Phases of Matter - PowerPoint PPT Presentation
Title:
Phases of Matter
Description:
Phases of Matter Four States Solid, Liquid, Gas, Plasma Matter Matter Matter Solids are incompressible Gases are compressible Liquids are very slightly compressible ... – PowerPoint PPT presentation
Number of Views:155
Avg rating:3.0/5.0
Title: Phases of Matter
1
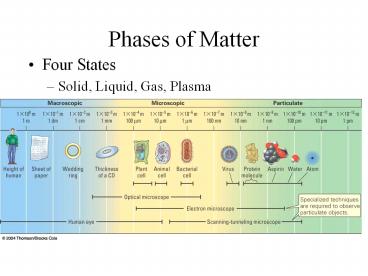
Phases of Matter
- Four States
- Solid, Liquid, Gas, Plasma
2
Matter (next unit)
- Phases of Matter
- Solids
- Has definite shape and definite volume
- Does not take the shape of the container and does
not take the volume of the container - Atoms or molecules are well ordered into a
crystalline lattice for most materials - Exceptions are things like glass which is really
a very slow flowing liquid
3
Matter
- Liquids
- No definite shape and has a definite volume
- Takes the shape of container and does not take
the volume of the container - Pretty incompressible
- Atoms or molecules loosely bonded to neighbors,
but in close proximity - Allows fluid to flow
4
Matter
- Gasses
- No definite shape and no definite volume
- Take the shape of the container and takes the
volume of the container - Compressible
- Atoms or molecules are widely separated and have
little interaction with each other aside from
elastic collisions
5
Matter
6
Matter
7
Matter
- Solids are incompressible
- Gases are compressible
- Liquids are very slightly compressible usually
considered incompressible
8
Matter
- Which of the following does not describe the
solid state? - a. Rigid, fixed, constant shape
- b. Constant volume
- c. Easily compressed
- d. Particles vibrate in fixed position
- e. All describe the solid state
9
Matter
- Which of the following does not describe the
liquid state? - a. Particles vibrate in fixed position
- b. Same shape as the bottom of the container
- c. Constant volume
- d. Pourable
- e. All describe the liquid state
10
Matter
- Which of the following does not describe the
gaseous state? - a. Same shape as a closed container
- b. Same volume as a closed container
- c. Random, independent particle movement
- d. Easily compressed
- e. All describe the gaseous state
11
Matter
- Plasma
- Temperature is so high that outer electrons are
separated from their underlying atoms - No molecules exist
- What is a molecule
- Molecule - neutral group of atoms united by
covalent bonds - Covalent bond - chemical bond resulting from the
sharing of electrons between two bonding atoms - Particles are electrically charged
- Sun, stars, inside a fluorescent bulb
12
Matter
- Mass
- Matter - anything that has mass and occupies
space - Mass - measure of the quantity of matter
- Weight is different.
- Weight is the force of gravity on the quantity of
matter - Weight changes with location moon vs. earth
- Varieties of matter
- Characteristics
- Pure sample pure sample of the same
- Substance - matter which has the same
characteristics or properties
13
Matter
- Element
- Can not be broken down into other substances by
ordinary chemical means - Periodic table of elements
- 91 elements found in nature
- The rest synthesized in a lab
- Compounds
- Substance made of two or more elements chemically
combined - Compounds can be decomposed into simpler
substances - Combined in definite proportion by mass
- The chemical and physical properties are
different from those of its constituents
14
Matter
- Compounds can be formed from simpler substances
by chemical change, and they can be decomposed
into simpler substances by chemical change - Math problems
- In every 100 g sample sample of water 11.2 g is
hydrogen and 88.8 g is oxygen. How many grams of
hydrogen is in a 140.0 g sample of water? - Sucrose (table sugar) is 42.1 carbon ( C ), 6.4
hydrogen (H), and 51.5 oxygen. How many grams
of C do you consume when you eat 50 g of sugar?
15
Matter
- Mixtures
- Consists of two or more substances, each which
retains its individual properties - Does it separate into 2 or more layers?
- Does it have to separate into 2 or more layers?
- Examples
- Element with element
- A compound is mixed with one or more compound(s)
- One or more element(s) with one or more
compound(s) - Characteristics
- Mixture retains properties of constituents
- Homogeneous mixture - prefix homo same
- Heterogeneous mixture - prefix hetero different
16
(No Transcript)
17
Matter
- Physical and Chemical Properties
- Physical - Characteristics that can be observed
w/o production of a new substance - Color
- Odor
- Taste
- Hardness
- Density
- Melting point (MP) and Boiling point (BP)
- Metals special characteristics
- Malleable- hammered into sheets
- Ductile drawn into wires
- Luster shine of metal
18
Matter
- Chemical- interaction with other substances to
produce new substances - Iron rust
- Nitrogen does not interact w/ most things as a
gas - Physical and Chemical Change
- Physical change one or more physical properties
of a substance are changed w/o change in the
substances chemical properties or composition
19
Matter
- Chemical change change that results in the
production of one or more substances that differ
in chemical properties and composition from the
original substance - Change in energy every change in matter
involves an energy change - Physical energy change is not as noticeable
- crystallization, freezing high energy release
- melting, evaporation low energy
- Chemical energy change more noticible
- explosions, burning high energy
- formation of hydrogen gas, oxygen gaslow energy
20
Energy and Phase Change
21
MatterEnergy and Phase Change
Adding heat at a constant rate
T
Time
22
Matter
Energy and Phase Change
Adding heat at a constant rate
T
Boiling
Melting
Time
23
Matter
- Conservation of mass
- Law of conservation of mass
- Matter can not be created or destroyed
- For ordinary chem change this holds true
- Lavoisier closed container no change,
open container change - Relative abundance
- Free state/Elemental state elements alone or
uncombined - Combined state elements combined w/ other
elements (most elements occur in nature only in
combined state) - Symbols of the elements chemistry reference
table pg. 11-12
24
Matter
- States of matter
- For a given substance, all four states are
possible - Increase temperature and a PHASE CHANGE occurs
- Solid gt Liquid gt Gas gt Plasma
25
Matter
- Phase changes
- Evaporation
- Condensation
- Boiling
- Melting
- Freezing
26
Water Molecule
- Two Hydrogen Atoms, One Oxygen Atom
-
-
27
Evaporation
28
Matter
- Evaporation
- Liquid cools because it loses higher energy
molecules or atoms - Air cools because the molecules leaving give up a
lot of energy to break free, so they have much
lower energy after they gain their freedom - Solid gt Gas directly is sublimation
29
Condensation
30
Condensation
- Condensation
- The opposite of evaporation
- Evaporation and Condensation are a SURFACE
phenomenon - Interaction of molecules at the liquid-air
interface
31
Boiling
- Molecules break free of the liquid bonds and form
little bubbles - If the vapor pressure in the bubble is large
enough to resist the pressure of the liquid, and
the air pressure above the interface they rise
and escape to the outside - Remember, liquid is cooling and air is cooling
32
Boiling
33
Boiling
34
Melting and Freezing
- Change between liquid and solid
- Bonds between molecules break when solid melts
- Takes energy to break the bonds
- Freezing is the opposite of melting
- Molecules move slowly enough that bonds can form
35
Melting and Freezing
- Why does salt melt ice?
36
Energy and Phase Change
For Pure Water
334 joules/gram
2256 joules/gram
37
Boiling Potatoes
- So, when you want garlic mashed potatoes and you
take potatoes and cut them up and put them in
boiling water, do they cook faster when the water
is really bubbling compared to when the water is
just barely bubbling???
38
Boiling Potatoes