Chapter 7 Electrochemistry - PowerPoint PPT Presentation
1 / 20
Title: Chapter 7 Electrochemistry
1
Chapter 7 Electrochemistry
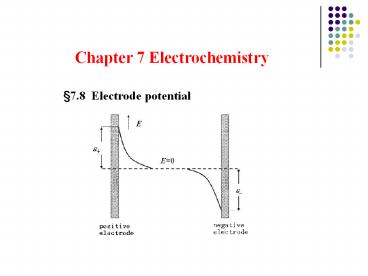
7.8 Electrode potential
2
How does electrode potential establish?
electromotive forces potential difference
Daniell cell
3
7.8.1. Interfacial charge and electrode
potential
1) Metal-metal interface contact
potential
2) Liquid-liquid interface Liquid junction is
the interface between two miscible electrolyte
solutions.
KCl solution HCl solution
liquid junction potential, liquid potential,
diffusion potential
4
3) Liquid-metal
exchange current, electrode potential
5
7.8.2. Models of electric double layer
3) Stern double layer (1924)
- Holmholtz double layer (1853)
2) Gouy-Chappman layer (1910, 1913)
Compact double layer
Diffuse double layer
6
7.8.3 Electromotive forces and relative electrode
potential
Cu(s)?Zn(s)?ZnSO4(m1)?CuSO4(m2)?Cu (s)
anode
cathode
E ?c ?? ?j ?
E ?c (?l,1- ??) (? - ?l,2) ? - ??
(?c ? l,1- ? l,2)
When emf of a cell was measured, we , in fact,
measured the potential difference between the two
electrodes.
7
Absolute potential
potentiometer
arbitrary reference
Can the absolute potential of electrode be
unmeasured?
Only the difference between two electrodes, i.e.,
electromotive of the cell E ?? ?? can be
measured.
8
(2) Normal/Standard Hydrogen Electrode (NHE/SHE)
In 1953, IUPAC defined normal hydrogen
electrode (NHE) as the reference for measurement
of electrode potential. IUPAC conventions
pure hydrogen gas at standard pressure
platinized platinum foil electrode
acidic solution with activity of H equals to 1.
definition
?? H/H2 0.000000 V.
9
(3) standard electrode potential
The potential of other electrode can be
obtained by combination of NHE and any other
unknown electrode into an electrochemical cell
with NHE serving as negative electrode and the
unknown electrode as positive electrode
- NHE unknown electrode
The sign and the value of the emf of the cell is
thus the sign and value of the potential of the
unknown electrode. All standard electrode
potentials are reduction potentials.
Cf. Levine, p. 431-435
10
Example
NHE ?? Cu2 (a0.1)?Cu
E 0.342 V
the electrode potential of the CuCu2 (a0.1)
electrode at pressure p and temperature T is thus
Because the unknown electrode is always
arranged as positive electrode, the electrode
reaction is, therefore, written in reduction form.
(reduction)(standard) electrode potential
11
(4) Nernst equation for electrode
SHECu2 (a0.1)Cu
Cell reaction H2(g, p?) Cu2(a) 2H (a1)
Cu
12
7.8.4 Reference electrode
Problems with NHE (primary standard) 1)The
platinized platinum electrode is easily poisoned
by adsorption of impurities from the solution and
the gas. 2) An elaborate purification is
required to purify the hydrogen before it is
passed through the cell. 3) Changes in
barometric pressure or in the depth of immersion
of the electrode in the solution produce a small
variation in the potential of the electrode. 4)
The preparation and the maintenance of the unit
activity solution are both much complicated.
13
Some electrodes with stable potential usually
used as the secondary standard, named as
reference electrode
The calomel electrode Hg(l)?Hg2Cl2(s)?KCl
(m)
??(T)/V 0.2412 - 6.61? 10-4 (T/?-25) - 1.7 ?
10-6 (T/?-25)2 - 9? 10-10 (T/?-25)3
saturated calomel electrode (SCE)
14
Other common reference electrodes
mercury-mercurous sulfate electrode Hg(l)?Hg2SO4(
s)?SO42?(m) ?? 0.640 V
Ag/Ag
mercury-mercuric oxide electrode
Hg(l)?HgO(s)?OH?(m) ?? 0.098 V
silver-silver chloride electrode
Ag(s)?AgCl(s)?Cl?(m) ?? 0.197 V
??(Ag/Ag) 0.799 V vs NHE ??(Ag/Ag)
__?___ V vs SCE
15
7.8.5. liquid junction potential and salt bridge
1) liquid junction potential
The diffusion of ions is irreversible, which
destroys the reversibility of the cell.
The value of Ej can reach ca. 30 mV, which is
too large for the measurement of emf.
16
2) influential factor of Ej
Pt(s), H2(g,p)?HCl(m)?HCl(m')?H2(g, p), Pt(s)
Ej
On passage of 1 mole of electrons through the
cell, t mol H and t? mol Cl? pass the boundary
For uni-univalence electrolytes
17
3) Effects of salt bridge
Every measurement of emf of a cell whose two
electrodes require different electrolyte raises
the problem of the liquid junction potential.
The problem can be solved either by measuring the
junction potential or eliminating it. The salt
bridge is often used to connect the two electrode
compartments to reduce the junction potential.
18
4) Salt bridge electrolyte
- does not react with either solution
- transference number of cation and anion is close
- of high concentration.
ions K NH4 Cl? NO3?
102 /Sm2mol-1 0.7352 0.734 0.7634 0.7144
t of some common salt bridge electrolytes
c/moldm-3 0.01 0.05 0.10 0.20
KCl 0.490 0.490 0.490 0.489
NH4Cl 0.491 0.491 0.491 0.491
KNO3 0.508 0.509 0.510 0.512
19
Concentration-dependence of Ej
c/moldm-3 0 0.2 1.0 2.5 3.5
Ej 28.2 19.95 8.4 3.4 1.1
Why does salt bridge reduce the junction
potential.
20
5) Effects of salt bridge
6) Elimination of junction potential