Crest - PowerPoint PPT Presentation
1 / 159
Title: Crest
1
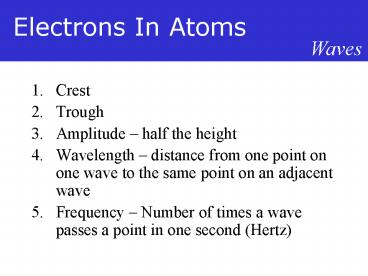
Waves
- Crest
- Trough
- Amplitude half the height
- Wavelength distance from one point on one wave
to the same point on an adjacent wave - Frequency Number of times a wave passes a point
in one second (Hertz)
2
(No Transcript)
3
Waves
- Frequency Wavelength
- Frequency Energy
- Wavelength Energy
- Amplitude Energy -
4
(No Transcript)
5
(No Transcript)
6
(No Transcript)
7
(No Transcript)
8
(No Transcript)
9
(No Transcript)
10
- How many complete waves are shown above?
- What is the wavelength of light shown above?
11
(No Transcript)
12
- Blu-Ray 405 nanometers (blue light)
- DVD 650 nanometers (red light)
13
- Calculate the number of wavelengths for each wave
shown to the left. - Calculate the wavelength of each wave.
- 1 nm 1 X 10-9 m. Convert each wavelength to
nm. - Which of the waves would be in the visible range?
14
Light
- All electromagnetic radiation moves at speed of
light (186,000 mi/s or 3 X 108 m/s) - All EM radiation is a form of light
- Visible light 400 nm to 700 nm
- violet red
15
The Electromagnetic Spectrum
Light
Safe radiation (non-ionizing)
Dangerous (ionizing)
Radio Radar Micro IR Visible Light UV X-rays Gamma
Produced by nuclear decay
16
(No Transcript)
17
(No Transcript)
18
- Microwaves
- Traditional Heat increase translational motion
of water - Microwaves increase rotational motion of water
19
Traditional Heat Microwaves
20
(No Transcript)
21
(No Transcript)
22
(No Transcript)
23
Light
24
(No Transcript)
25
(No Transcript)
26
- c ln
- c speed of light (3 X 108 m/s)
- l wavelength (meters)
- frequency (Hz or s-1)
- Important conversion 1 nm 1 X 10-9 m
27
- Calculate the wavelength of a 60 Hz EM wave
- 5 X 106 m
28
- Calculate the wavelength of a 98.5 MHz FM radio
station - 3.05 m
29
- Calculate the frequency of 500 nm blue light.
- 6 X 1014 s-1
30
Light
- 6. Wave-Particle Duality
- a. light can be viewed as both a wave and a
particle - b. Max Planck/Einstein 1910
- c. Photon has no mass, only energy
31
Light
32
- E hn (for one photon)
- E Energy (J)
- h 6.63 X 10-34 J s (Plancks constant)
- n frequency (Hz)
33
- Calculate the energy of laser light with a
frequency of 4.69 X 1014 s-1 . - Ans 3.11 X 10-19 J (This is for one photon)
34
- Calculate the energy of a photon of wavelength
600 nm. - ANS3.3 X 10-19 J
35
- Calculate the energy of a photon of wavelength
450 nm (blue light). - Ans 4.42 X 10-19 J (This is for one photon)
36
- A single photon has an energy of 3.616 X 10-19
J. - Calculate the frequency of the photon.
- Calculate the wavelength of a photon in meters
- Calculate the wavelength of a photon in
nanometers. - Is this photon in the visible range?
- What range of the spectrum would you expect a
photon of 800 nm to be? - Calculate the energy for one mole of photons with
individual energies of 3.616 X 10-19 J.
37
- 5.45 X 1014 Hz
- 5.50 X 10-7 m
- 550 nm
- Yes
- IR
- 3.616 X 10-19 J X 6.02 X 1023 photons
- 1 photon 1 mole
- 2.18 X 105 J/mol
38
Newtonian Mechanics Quantum Mechanics
Everything is a particle Everything is both a wave and a particle
Large objects (dust, people, baseballs, etc..) Photons, electrons, atoms, molecules
All values are allowed Quantized only certain values allowed
Predictable Probabilistic
- My l 8.1 X 10-36 m at 3 mph
39
(No Transcript)
40
Bohr Model
- Neils Bohr Planetary Model
- Studying line spectra of elements
- Only certain lines are present (quantized)
- Not a rainbow
- Spectra are a fingerprint for atoms/molecules
(Astronomy)
41
(No Transcript)
42
Quantized only certain orbits exist (rest is
forbidden zone)
43
(No Transcript)
44
(No Transcript)
45
- 4. Ways to make something glow
Bohr Model
Photon Absorption Collision -Glow in the
dark -Heat -Electricity -Chemical
Reaction
46
Photon Absorption Collision
47
(No Transcript)
48
(No Transcript)
49
- A single photon has a wavelength of 150 nm.
- Calculate the wavelength of a photon in meters
(1.50 X 10-7 m) - Calculate the frequency of the photon. (2.0 X
1015 Hz) - Calculate the energy of the photon. (1.33 X 10-18
J) - Is this photon in the visible range?
- Calculate the energy for one mole of these
photons. (8.01 X 105 J)
50
Quantum Mechanical Model
- Electron as a particle
- Heisenberg Uncertainty Principle can never know
both the position and velocity of an electron at
the same time
51
a. Electron cloud b. Electron moves randomly
(not like a planet) c. Orbital region of 90
probability
52
nucleus
Random electron cloud
53
Quantum Mechanical Model
- Electron as Wave
- Schrodinger Wave Equation (1926) treats
electron solely as a wave
54
(No Transcript)
55
(No Transcript)
56
Quantum Mechanical Model
- Result One - Explains the forbidden zone (waves
do not match)
57
Quantum Mechanical Model
Waves match here (get a clear note)
Waves do not match here (get a bad note,
forbidden zone)
58
Quantum Mechanical Model
- Result Two
- Orbits are not circular
59
Bohr Model Heisenberg (Particle) Schrodinger (Wave)
Explains line spectra Planetary model Electron moves randomly Electron cloud Explains f. zone Shapes of orbits
60
- Draw an s, p and d orbital
- How many electrons can be placed in an s orbital?
- How many electrons can be placed in an p orbital?
In a p suborbital? - How many electrons can be placed in an d orbital?
In a d suborbital? - How many electrons can be placed in an f orbital?
In an f suborbital? - How did Heisenberg consider the electron?
- How did Schrodinger consider electron?
61
Quantum Numbers
- First QN how far the electron is from the
nucleus (larger the number, farther away) Level
or shell
n 2
n 1
62
Quantum Numbers
- Second QN the shape of the orbital
63
Quantum Numbers
- Third QN the suborbital
- Orbital suborbitals Total e-
- s 0 2
- p 3 (px,py,pz) 6
- d 5 10
- f 7 14
64
(No Transcript)
65
(No Transcript)
66
Quantum Mechanical Model
67
(No Transcript)
68
(No Transcript)
69
Quantum Numbers
- Fourth QN spin of the electron
- Pauli Exclusion Principle two electrons in the
same suborbital (ex px) must have opposite
spins - 1/2 -1/2
70
(No Transcript)
71
(No Transcript)
72
(No Transcript)
73
(No Transcript)
74
(No Transcript)
75
Electron Configuration
76
Electron Configurations
- Electron Configuration shorthand notation to
tell you the locations of all the electrons in an
atom or ion - Notation
- 2p3
- Orbit Shape e-
77
Electron Configurations
78
(No Transcript)
79
Electron Configurations
- H
- He
- Li
- O
- Fe
- S
80
- Be V
- N F
- Sr Ar
- P Mg
- Se Kr
81
- Which element is represented by the following
electron configurations? - 1s22s22p63s23p64s23d5
- 1s22s22p63s23p64s23d104p65s24d7
- 1s22s22p63s23p64s1
- 1s22s22p63s23p3
- 1s22s22p63s1
- 1s22s22p63s23p2
- 1s22s22p63s23p64s23d104p6
82
Noble Gas Shortcut
- Rule Use the noble gas in the previous row
- Examples
- Ne
- P
- Ru
- Kr
- You try
- Br Ar S Ca I Xe
83
Br I
Ar Ca
S Xe
84
Exceptions
- Mostly with transition metal elements
- There is a special stability to filled and
half-filled orbitals
Element Actual configuration Instead of
Cr Ar4s13d5 Ar4s23d4
Mo Kr5s14d5 Kr5s24d4
Cu Ar4s13d10 Ar4s23d9
Ag Kr5s14d10 Kr5s24d9
85
Ions
- p e e- configuration
- Sr
- Sr
- Sr2
86
Ions
- p e e- configuration
- S
- S1-
- S2-
- Br1-
- Ba2
87
p e E configuration
Na
P3
P3-
Sn2
B3
Se2-
Cl-
As3-
88
- F1-
- Ca2
- N2
- S2-
- As3-
89
Valence Electrons
- Outershell Electrons
- Only Electrons involved in bonding
- H2O example
- Many elements want 8 valence electrons (Noble Gas
Configuration)- Full Octet
90
(No Transcript)
91
Valence Electrons
- e config ve Lewis dot
- H
- Li
- Be
- Mg
92
Valence Electrons
- e config ve Ldot
- O
- S
- C
- Ge
93
Valence Electrons
- e config Ldot
- Na
- Na
- Mg
- Mg
- Mg2
94
- e config Ldot
- B
- B1
- B2
- B3
- Te
- Te1-
- Te2-
Valence Electrons
95
Valence Electrons
- e config Ldot
- Be
- Be
- Be2
96
Valence Electrons
- e config ve Ldot
- Cl
- Cl-
- O
- O1-
- O2-
97
Gr I Gr II Gr III Gr IV Gr V Gr VI Gr VII Gr VIII
1 v. e- 2 v. e- 3 v. e- 4 v. e- 5 v. e- 6 v. e- 7 v. e- 8 v. e-
1 2 3 No charge -3 -2 -1 0
98
Periodic Properties
- Periodic Properties Properties that depend on
an elements position on the table - Ex Groups
- H, Li, Na all form similar oxides
- (H2O, Li2O, Na2O)
- Location gives you A LOT of information
99
(No Transcript)
100
Size of Atoms
- Atomic Radius
- 1. Measured in
- picometers (1pm 1 X 10-12 m) or Angstroms
(1 Å 100 pm) - 2. Average radius 100 pm (1 Å)
101
(No Transcript)
102
Size of Atoms
- 3. Example Bromine 1.14 Å
- 1.14 Å X 100 pm 114 pm
- 1 Å
103
- Effective Nuclear Charge
- Charge from nucleus that
- is not blocked (shielded)
- by core electrons
- Zeff Z-S
- Z protons
- S core electron
104
- What is the Zeff for Lithium (1s22s1)?
105
- What is the Zeff for Fluorine (He2s22p5)?
106
- e- configuration Zeff
- S
- O
- P
- O2-
- Mg2
- K
107
Size of Atoms
- Down a group
- e- config Levels Zeff
- H
- Li
- Na
108
Size of Atoms
- Down a group atoms get larger, more levels
- e- config Levels Zeff
- H
- Li
- Na
109
Size of Atoms
110
Size of Atoms
- Across a period atoms get smaller. Same levels,
greater Zeff (nucleus pulls electrons closer) - Li F
- E config
- levels
- Zeff
111
Size of Atoms
112
Size of Atoms
- Si Cl
- E config
- levels
- Zeff
113
Size of Ions
- A. Positive Ions
- 1. Example
- Mg Mg Mg2
- E config
- levels
- Zeff
- electrons
114
Size of Ions
115
Size of Ions
- Positive ions always smaller
- Fewer electrons to control
- Less e- to e- repulsion
116
- Mg Mg Mg2
- E config
- levels
- Zeff
- electrons
117
Size of Ions
- B. Negative Ions
- 1. Example
- O O2-
- E config
- levels
- Zeff
- electrons
118
Size of Ions
119
Size of Ions
- Negative ions always larger
- More electrons to control
- More e- to e- repulsion
120
- Rank the three elements from smallest to largest
- Which factor is most important in comparing Mg
and Sr, levels or Zeff? Explain. - Which factor is most important in comparing Mg
and S, levels or Zeff? Explain. - Which would be larger, S or S2-? Explain.
Mg S Sr
Electron Config.
Levels
Zeff
121
More levels
If same
Greater Zeff (same levels, greater Zeff smaller)
If same
Ions Positive Smaller(less electron
repulsion) Negative Larger (more electron
repulsion)
122
Size Review
- Which is larger and why?
- Li or K
- S or S2
- Mg or S
- O or Te
123
Size Review
- Which is larger and why?
- Cl or Al
- B or B
- Al or In
- B or B-
124
Size Review
- Kurveball
- K or K
125
Ionization Energy
- A.Ionization energy The energy needed to remove
an electron from an atom - Na ? Na e-
126
A high energy photon may ionize an atom
(completely remove the electron)
A low energy photon will excite an electron
127
He
Ne
Ar
H
Li
Na
K
128
Ionization Energy
- B. Across a period Ionization Energy
INCREASES - 1. Harder to remove an electron (atom is
smaller, holds e- more tightly) - 2. Examples
- Li (520 kJ/mol) F (1681)
129
(No Transcript)
130
Ionization Energy
- C. Down a groupIonization Energy DECREASES
- 1. Easier to remove an electron (atom is
larger, holds e- more loosely) - 2. Examples
- Li (520 kJ/mol)
- Na (496 kJ/mol)
- K (419 kJ/mol)
131
Ionization Energy
- Which has the higher Ionization Energy and why?
- C or O
- Na or Cl
- C or Sn
- Mg or Ra
132
Multiple Ionization Energy
- Multiple Ionizations - Removing more than one
electron - 1st Mg ? Mg e- 738 kJ/mol
- 2nd Mg ? Mg2 e- 1450 kJ/mol
- 3rd Mg2 ? Mg3 e- 7732 kJ/mol
- There is a large jump once you reach Noble Gas
Configuration (Fewer levels, spike in Zeff)
133
Multiple Ionization Energy
134
(No Transcript)
135
Multiple Ionization Energy
- 1st Al ? Al e- 577 kJ/mol
- 2nd Al ? Al 2 e- 1816 kJ/mol
- 3rd Al 2 ? Al 3 e- 2744 kJ/mol
- 4th Al3 ? Al4 e- 11580 kJ/mol
136
Multiple Ionization Energy
- Examples
- a. Where will the large jump in I.E. occur for
- Be B P
- b. Element X has a large jump between its 4th
and 5th I.E. To what group does it belong?
137
Size Review
- Which is larger and why?
- N or N3-
- C or F
- Sr or Be
- O or O2-
138
Size Review
- Which has the larger ionization energy and why?
- P or P3
- B or F
- Ba or Be
- S or Na
139
Light
- Spectroscopy
- Spec 20
- a. Light Source
- b. Slit
- c. Prism/Monochromator
- d. Sample
- e. Light Meter (PMT)
140
- Emission Spectrum of Air
141
Wavelength(nm) Wavelength(m) Frequency (Hz) Energy (J)
550 5.50 X 10-7 5.45 X 1014 3.61 X 10-19
120 1.20 X 10-7 2.50 X 1015 1.66 X 10-18
0.115 1.15 X 10-10 2.61 X 1018 1.73 X 10-15
1490 1.49 X 10-6 2.01 X 1014 1.33 X 10-19
405 4.05 X 10-7 7.41 X 1014 4.91 X 10-19
650 6.50 X 10-7 4.62 X 1014 3.06 X 10-19
800 8.00 X 10-7 3.75 X 1014 2.49 X 10-19
14.9 1.49 X 10-8 2.01 X 1016 1.33 X 10-17
142
- 2a. 1 X 10-7 m
- 3 X 1015 Hz
- 1.99 X 10-18 J
- 400-700 nm
- UV
- 3a. 6 X 10-7 m
- b. 600 nm
- c. 3.31 X 10-19 J
- Red
- Longer
143
- Page 231 Assessing
- 8-2 a
- Orbital
- s, p, d, f
- spherical
- p-orbital
- 3 subshells
- p orbitals
- 8-3 a) 18 b) 3 c) 2, 6, 10 d) 0,3,5
144
- 8-1 UV light has higher energy (shorter
wavelength) - 8-2 n9 to n1 will have a shorter wavelength
- 8-3 n3 to n1 will have a shorter wavelength
- 8-4 Excited state, Li is only in the second
period - 8-5 1p and 3f
- 8-6 2d does not exist
145
- Li 1s22s1
- Br 1s22s22p63s2 3p64s23d104p5
- In 1s22s22p63s2 3p64s23d104p65s2 4d105p1
- Ne 1s22s22p6
- N 1s22s22p3
- Ca 1s22s22p63s2 3p64s2
- Al 1s22s22p63s2 3p1
- S 1s22s22p63s2 3p4
- Kr 1s22s22p63s2 3p64s23d104p6
- Zr 1s22s22p63s2 3p64s23d104p65s2 4d2
- Fe 1s22s22p63s2 3p64s23d6
146
- C 1s22s22p2
- Ar 1s22s22p63s2 3p6
- Pd 1s22s22p63s2 3p64s23d104p65s2 4d8
- He 1s2
- O 1s22s22p4
- Ti 1s22s22p63s2 3p64s23d2
- Na 1s22s22p63s1
- Mg 1s22s22p63s2
- Si 1s22s22p63s2 3p2
- C 1s22s22p2
147
- Ne He2s22p6 S Ne3s23p4
- Si Ne3s23p2 In Kr5s24d105p1
- Sr Kr5s2 K Ar4s1
- Fe Ar4s23d6 Cu Ar4s23d9
- Te Kr5s24d105p4
- P Ne3s23p3
- N He2s22p3
- Ni Ar4s23d8
- Br Ar4s23d104p5
- Be He2s2
148
- Ne3s2 Mg
- Ar4s23d3 V
- Kr5s24d105p5 I
- Ar4s23d104p6 Kr
- He2s22p6 Ne
- Ne3s23p5 Cl
149
- O He2s22p4 Al Ne3s23p1
- O1- He2s22p5 Al Ne3s2
- O2- He2s22p6 Al2 Ne3s1
- Mg Ne3s2 Al3 He2s22p6
- Mg1 Ne3s1 Cl Ne3s23p5
- Mg2 He2s22p6 Cl1- Ne3s23p6
- N He2s22p3 Cl3 Ne3s23p2
- N3- He2s22p6
- N3 He2s2
- N5 1s2
150
- Excited state, can emit a photon
- 6. 2d does not exist (ds start with 3d)
- Area of space where an electron is likely to be
found - 4 lobes (eggs), 4p has only 2 eggs
- a) Tl b) Y c) Ce d) As
- 141 pm Sn 180 pm Tl
151
- Ba(NO3)2
- N2O4
- Fe2(SO4)3
- copper(II) chloride
- nitrogren trihydride
- Aluminum hydroxide
152
- 1,1
- 1,2 2,1
- 1,3 3,1 2,2
- 1,4 4,1 2,3 3,2
- 1,5 5,1 2,4 4,2 3,3
- 1,6 6,1 2,5 5,2 4,3 3,4
- 2,6 6,2 3,5 5,3 4,4
- 3,6 6,3 4,5 5,4
- 4,6 6,4 5,5
- 5,6 6,5
- 6,6
153
- a) As b) Ru c) Ba d) I
- a) Tl b) Y c) Ce d) As
- Cr 117 pm, Nb 134 pm
- Sn 141 pm, Tl 180
- a) V b) Cl c) Mg d) Fe e) B
154
- Ca More levels
- Br- - Same levels and Zeff, more e- to control
- Mg - Same levels, lower Zeff
- Sb - More levels
- Li More levels
- Sn More levels
- F Smaller atom (same levels, higher Zeff)
- C Smaller atom (fewer levels)
- Mg - Smaller atom (same levels, fewer electrons)
- S - Smaller atom (same levels, higher Zeff)
- Al3 Smaller atom (fewer levels)
155
- Sr More levels
- Sr Same levels, Sr has a lower Zeff
- Sn Same levels, same Zeff, Sn has more e to e
- repulsion
- Te2- Same levels, same Zeff, Te2- has more e to
e - repulsion
- Fr More levels
- Al2 More levels
156
- K Smaller atom, holds electrons more tightly
- Cl Smaller atom, holds electrons more tightly
- Ba2 Smaller atom, holds electrons more tightly
- Al Between 3rd and 4th
- Rb Between 1st and 2nd
- Ra Between 2nd and 3rd
157
Answers to Review Test
1 C 11 D
2 B 12 B
3 A 13 A
4 B 14 C
5 A 15 D
6 D 16 E
7 B 17 D
8 A 18 C
9 C 19 B
10 B 20 D
158
- E config L. Dot
- S
- S1-
- S2-
- Na
- Mg2
159
(No Transcript)