Antibodies (Chapter 4) - PowerPoint PPT Presentation
1 / 30
Title:
Antibodies (Chapter 4)
Description:
Title: PowerPoint Presentation Subject: The Immune System Author: Parham Last modified by: student Created Date: 12/16/2002 8:36:41 PM Document presentation format – PowerPoint PPT presentation
Number of Views:252
Avg rating:3.0/5.0
Title: Antibodies (Chapter 4)
1
Antibodies(Chapter 4)
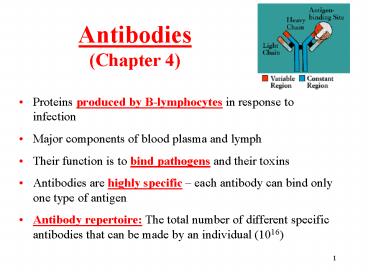
- Proteins produced by B-lymphocytes in response to
infection - Major components of blood plasma and lymph
- Their function is to bind pathogens and their
toxins - Antibodies are highly specific each antibody
can bind only one type of antigen - Antibody repertoire The total number of
different specific antibodies that can be made by
an individual (1016)
2
Antibodies are the secreted form of
immunoglobulins Plasma cells release antibody of
the same antigen specificity as the membrane
bound immunoglobulin expressed by their B-cell
precursor.
3
Antibodies are glycoproteins built from two
identical heavy chains (H-chains) and two
identical light chains (L- chains). Carbohydrate
is attached to the H-chains. Antibodies have
characteristic Y-shaped structure. There are 5
different classes of antibodies (Ig) IgA, IgD,
IgE, IgG, and IgM. IgG the most abundant Ig. Mw
of IgG is 150 kD (50 kD for H-chain and 25 kD for
L-chain).
Antibody structure
4
Antibodies are composed of polypeptides with
variable and constant regions
The N-terminal regions (called variable regions
V-regions) vary greatly in amino-acid sequences,
and are responsible for the antibody specificity,
and binding to the antigen. The remaining parts
have more conserved amino-acid sequences, and are
called constant (C-regions). They are responsible
for binding to other immune cells.
VH VL
5
The Y-shaped Ig molecule can be cleaved by
proteases
Fragment antigen binding Fragment crystallizable
- Flexible hinge region in the middle of the heavy
chain - Can be cleaved by different proteases
6
Antibody structure
Arms of Ig are called Fab Stem of Ig is called Fc
region
Hinge region is the region at which the arms of
the antibody molecule forms a Y. It is called the
hinge region because there is some flexibility in
the molecule at this point.
7
Differences in the heavy C-regions define five
main isotypes (classes) of Ig IgA, IgD, IgE, IgG
and IgM
- The isotypes differ in
- Length of the heavy C-regions
- Location of disulfide bonds linking the chains
- Hinge region (present in IgG, IgD and IgA, and
absent in IgM and IgE) - Distribution of carbohydrate groups
- In the membrane-bound form, all Igs are
monomers. - In the secreted form ( antibodies), IgD, IgE
and IgG are monomers IgM is a pentamer and IgA
can exist as a monomer or a dimer.
8
Two isotypes of the light chain
- Kappa (k) 2/3 of antibody molecules in humans
- Lambda (l) 1/3 of antibody molecules in humans
- No functional difference
- Each antibody contains either k or l
9
Immunoglobulin chains are folded into compact
and stable protein domains
Heavy and light chains consist of motifs of
100-110 amino acids that are folded into compact
domains, called immunoglobulin domains. The light
chain has one variable domain (VL) and one
constant domain (CL). The heavy chain has one
variable domain (VH) and three constant domains
(CH1, CH2, and CH3). VH and VL domains together
form the antigen-binding site.
10
Hypervariable regions
- The differences in amino acid sequence in the V
domains of heavy and light chains are
concentrated in hypervariable regions (HV), also
called complementarity-determining regions (CDR).
- HVs (CDRs) are flanked by much less variable
framework regions (FR). - Each V domain has three HVs and four FRs.
11
Antigen-binding sites are formed from the
hypervariable regions of VL and VH
The pairing of a heavy and a light chain brings
together the HV loops (CDRs), which form the
antigen-binding site.
12
Antigenic determinant (epitope)
- Individual antigens are usually composed of a
cluster of AA or a part of polysaccharide. - Epitope is the specific part of the antigen to
which the antibody binds - Antigen that contains more than one epitope
multivalent antigen. - There can be two forms of multivalent antigens
1) with different epitopes and 2) with multiple
copies of the same repeated epitope.
Two kinds of multivalent antigen
13
Chemical nature of antigens (epitopes)
- Antibodies can be made to any chemical structure
- Most often, antigens are carbohydrates or
proteins - In allergic reactions or autoimmune diseases,
antigens can be drugs (penicillin), environmental
substances (pollen), metals (allergy to
jewelry), DNA (lupus), or antibodies (arthritis).
14
Recognition of antigens (epitopes) by B-cell
receptors (BCR) is highly specific
15
Antibody binds to antigen by non-covalent forces
Antigen-binding sites of antibodies are usually
rich in aromatic AA (Phe, Tyr, Trp), which can
form strong hydrophobic interactions
16
Main characteristics of antibodies
- IgM The first antibody produced in response to
pathogen. By isotype switching, synthesis of IgM
gives a way to synthesis of IgG. - IgG The most abundant antibody. IgG is smaller
and more flexible than IgM. During pregnancy, it
can be transferred across placenta to provide the
fetus with protective antibodies from the mother. - IgA The main antibody in body fluids - tears,
sweat, breast milk and saliva. - IgE Highly specialized induces activation of
the mast cells involved in parasitic infections.
Responsible for allergies when produced against
harmless antigen. - IgD Present in serum in low amounts its
function is not fully understood.
17
Polyclonal vs. monoclonal antibodies
Polyclonals Monoclonals
Immunize animals (rabbits) with the appropriate antigen Prepare antisera from their blood IN VIVO The specificity of the antibody is highly dependent on the purity of the antigen ? Can be made only if antigen is available in highly purified form. Fuse B-cells producing antibodies with tumor plasma cells to form hybridomas Test the cells which produce the desired antibody, and clone them IN VITRO Does not require to have purified antigen. The antibodies produced by hybridomas are all identical (produced from the same clone).
18
POLYCLONAL ANTIBODIES
- Obtained by bleeding animal following response to
antigen. Usually 4-6 weeks after initial
injection (or immunization). Takes about 3 weeks
for initial immune response required to develop a
population of B-cells making high affinity IgGs.
- In response to antigen, B-lymphocytes will
differentiate and produce clones of cells that
make various IgGs that recognize the antigen.
Hence the term POLYCLONAL ANTIBODIES. Each
antibody has the potential to recognize different
sites on the molecule or the same site in
different ways. IgGs are secreted in blood. - Following bleeding of the animal, the serum (or
ANTISERUM) can be used directly for many
immunological assays. Antiserum will contain
many blood proteins and many IgGs in addition to
the ones elicited specifically against the
antigen of interest. (In fact, only 1-5 of the
IgG fraction will be against your antigen).
- ADVANTAGES AND DISADVANTAGES OF POLYCLONAL
ANTIBODIES - 1) Easiest and cheapest way to prepare
antibodies. Animal does all the work. - 2) Obtain many types of IgGs that
recognize the same protein. - 3) Antiserum only as good as the antigen
preparation you injected. If the antigen is
contaminated with other substances, antibodies to
those contaminants also maybe produced.
19
Production of monoclonal antibodies
Nobel Prize in Medicine in 1984 was awarded to
Milstein, Kohler, and Jerne for the discovery of
the technology to produce monoclonal antibodies
20
Production of monoclonal antibodies
Polyethylene glycol-mediated fusion of
antibody-producing cells with myeloma cells
21
Uses for MAbs Diagnosis
- Identification of tumors and classification of
leukemia - Screening for prostate cancer (PSA)
- Identification of pathogens
- HIV testing
22
Uses for MAbs Treatment
- To suppress the immune system
- Remicade Monoclonal antibody neutralizing TNF
used in rheumatoid arthritis
- Cancer immunotherapies
- Herceptin Binds protein (HER2) expressed on some
tumor cells (breast cancers, lymphomas) inhibits
cancer cell proliferation
23
Immunological methods based on antibodies
- ELISA
- Western blotting
- Immunofluorescence
- Immunoprecipitation
- Based on an antigen-antibody interaction used
in medicine and molecular biology
24
Enzyme-Linked Immunosorbent Assay (ELISA)
- Can be used to detect antigens or antibodies in a
sample
The higher the concentration of the antigen in
the sample, the higher the concentration of the
color product, and absorbance
25
Introduction to Western Blotting
- The term blotting refers to the transfer of
biological samples from a gel to a membrane, and
their subsequent detection on the surface of the
membrane. - Western blotting (also called immunoblotting
because an antibody is used to specifically
detect its antigen) was introduced by Towbin, et
al. in 1979, and is now a routine technique for
protein analysis. - The specificity of the antibody-antigen
interaction enables a single protein to be
identified in the midst of a complex protein
mixture. - Western blotting is commonly used to positively
identify a specific protein in a complex mixture
and to obtain qualitative and semiquantitative
data about that protein.
26
Immunoblotting (Western Blot)
- Procedure
- proteins separated by electrophoresis on a
protein SDS-gel - proteins transferred to (nitrocellulose or PVDF)
membrane sheets - protein bands visualized with enzyme-tagged
antibodies - Applications
- Diagnosis (diseases)
- Research (analysis of protein expression)
27
Western Blotting
- In Western blotting, proteins are first separated
by SDS electrophoresis (SDS-PAGE). - As the proteins migrate through the gel they are
separated based upon size and charge. - Characteristically, smaller proteins migrate
through the gel faster than larger proteins.
28
(No Transcript)
29
ELISA
Western Blotting
Time (h) 0 0.5 3 9 18
IL-8 Actin
Intracellular levels of IL-8 in human neutrophils
analyzed by western blotting
IL-8 release from prostate cancer cells measured
by ELISA
Confocal immunofluorescence
Intracellular localization of IL-8 in a single
human neutrophil analyzed by confocal
immunofluorescence microscopy
30
Antibodies Summary
- Antibodies are made of four polypeptide chains
two identical heavy chains and two identical
light chains. - Each antibody has a V region that contains the
antigen-binding site and determines specificity
of the antibody, and a C region, which determines
the antibody isotype and its effector functions. - In the V-domain, the sequence variability is
concentrated into three hyper-variable regions. - The type of antigen bound by an antibody depends
on the shape of the antigen-binding site each
antibody molecule has two antigen binding sites. - Monoclonal antibodies are antibodies of a single
specificity that originate from one clone of
identical antibody-producing cells. They are
produced by fusion of mouse plasma cells with
cancer cells, and are used in diagnostic tests
and as therapeutic agents.