Volumetric analysis - PowerPoint PPT Presentation
Title:
Volumetric analysis
Description:
Volumetric analysis To understand volumetric analysis, we must understand the types of reaction that happen in it. Types of reactions used in volumetric analysis : – PowerPoint PPT presentation
Number of Views:115
Avg rating:3.0/5.0
Title: Volumetric analysis
1
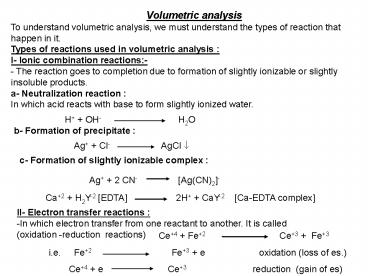
Volumetric analysis To understand
volumetric analysis, we must understand the types
of reaction that happen in it. Types of
reactions used in volumetric analysis I- Ionic
combination reactions- - The reaction goes to
completion due to formation of slightly ionizable
or slightly insoluble products. a-
Neutralization reaction In which acid reacts
with base to form slightly ionized water.
H OH-
H2O b- Formation of precipitate
Ag Cl- AgCl ?
c- Formation of slightly ionizable complex
Ag 2 CN-
Ag(CN)2-
Ca2 H2Y-2 EDTA 2H CaY-2
Ca-EDTA complex
- II- Electron transfer reactions
- In which electron transfer from one reactant to
another. It is called - (oxidation -reduction reactions)
Ce4 Fe2 Ce3
Fe3
i.e. Fe2 Fe3
e oxidation (loss of es.)
Ce4 e Ce3
reduction (gain of es)
2
Acid-Base Acid- base theories
1- Arrhenius theory - Acid Is the substance
which ionize to give H eg. HCl Base
Is the substance which ionize to give OH-
eg NaOH
2- Bronsted - Lowry theory - Acid Is the
substance which donate proton. Base Is the
substance which accept proton. Every acid has a
conjugate base and the base has conjugate
acid. The stronger the acid the weaker its
conjugate base and vice versa.
Eg. HCl H2O
Cl- H3O
Acid base
conj.base conj.acid
Eg. NH3 H2O
NH4 OH-
base acid
conj.acid conj.base N.B. Water
behave as acid or base because it is neutral.
3
3- Lewis theory - Acid Is substance which
accept lone pair of electrons eg. BF3,
AlCl3. Base Is substance which donate lone pair
of electrons eg NH3, amines.
Acid-base titration in aq. medium Solns. are
classified into - Electrolytes Which desociate
(ionize) and conduct electricity. or Non
electrolytes Which doesn't ionize and doesn't
conduct electricity.
Dissociation of water
H2O
H OH- Dissociation const. Kw H OH-
/ H2O - Since H2O is weakly dissociated ,
therfore H2O is considered unity. therfore Kw
H OH- 10 -14 at 25oc Kw it is called
ionic product of water. At 25oc H OH-
10-7 If H OH- , therfore soln. is
neutral If H gt 10-7 eg 10-6, 10-5 , therfore
soln. is acidic If H lt 10-7 eg 10-8, 10-9 ,
therfore soln. is alkaline.
4
Hydrogen exponent pH pH -log H i.e. If
H 10-7 pH - log 10-7 7 In acidic
side i.e. If H 10-6 pH - log 10-6
6 In basic side i.e. If H 10-8 pH -
log 10-8 8
i.e. as pH value inc. H conc.
decrease. Therefore acid soln has pH less than 7
, alkaline soln. has pH more than 7 and neutral
soln. has pH p OH 7
pH of acid and bases - 1- pH of strong acids
- Since strong acids are strongly
ionized. Therfore pH pCa
where Ca ( conc. of acid) i.e. 0.1 N
HCl pH - log 0.1 - log 10-1 1 2- pH of
strong bases - Since strong bases are completely
ionized. Therfore p OH p Cb
where Cb (conc.of base) pH p Kw p
OH i.e. pH p Kw p Cb. i.e. 0.1 N
NaOH pH 14 _ 1 13
3- pH of weak acids pH 1/2 pCa 1/2 pKa 4-
pH of weak bases - pH pKw - 1/2 pCb - 1/2 pKb
5
5- pH of salts - a- Salt of strong acid and
strong base eg. NaCl Always neutral i.e. pH
7 b- Salt of strong acid and weak base
eg.NH4Cl Always pH is in the acidic side ,
calculated from eq. pH 1/2 pKw - 1/2 pKb
1/2pCs where Kb (dissociation constant of weak
base) Cs (conc. of salt) c- Salt of weak acid
and strong base eg. Na Ac Always pH is in the
alkaline side, calculated from eq. pH 1/2 pKw
1/2 pKa - 1/2pCs where Ka (dissociation
constant of weak acid) Cs (conc. of salt) d-
Salt of weak acid and weak base eg. NH4Ac pH is
calculated from eq. pH 1/2 pKw 1/2 pKa -
1/2pKb
Buffer solutions -Def They are solns which
resist changes in pH upon addition of small
amount of acid or base. -They consist of weak
acid and its salt or weak base and its salt Type
1- weak acid and its salt eg. HAc and Na Ac pH
of this buffer is calculated from the eq. pH
pKa log salt / acid
6
Type 2- weak base and its salt eg. NH4OH and
NH4Cl pH of this buffer is calculated from the
eq. pH pKw - pKb - log salt/base log salt/acid
or log salt/base is called buffer
ratio if salt acid therefore pH
pKa Examples 1- Calculate the pH of a buffer
soln. containing 0.1 M acetic acid and 0.1 M
sodium acetate pKa 4.76 soln. pH pKa log
salt / acid pH 4.76 log 0.1 / 0.1 4.76
Neutralization indicators Color indicators -
Substance which change their color with change in
pH are used as neutralization indicators. eg.
phenol phthalein"Ph.Ph" (one color indicator),
methyl orange"M.O" (2 color indicator) eg.
Ph.Ph. 8-10 M.O. 3.3-4.4
M.R. 4-6 N.B. the
indicator is chosen according to pH of the
product.
7
Neutralization titration curves For
neutralization reaction titration . The titration
curve is plot of pH versus the mls of
titrant. Types of neutralization curves 1-
Strong acid -strong base titration - eg. HCl and
NaOH we have sample of 100 ml HCl and titrate
against NaOH.
- Before the titration pH is due to
- the sample i.e HCl (strong acid)
- therfore pH pCa
- b- At the equivalent point
HCl NaOH NaCl H2O pH is due to
NaCl i.e. pH 7 (salt of strong acid and
strong base) c- After the equivalent point pH
is due to excess titrant i.e. NaOH (strong base)
pH pKw-pCb N.B. The pH rises slowly till 99.9
of acid is titrated by adding 0.1 ml NaOH pH
rises from 4 to 7 then another 0.1 ml after end
point pH rises from 7 to 10 i.e. at e.p. pH
rises from 4 to 10. So we can use M.O.(3.3 -
4.4)- M.R.(4 - 6)- Ph.Ph.(8 - 10) indicators