Vacuum Systems - PowerPoint PPT Presentation
Title:
Vacuum Systems
Description:
UW- Madison Geology 777 Electron Probe Microanalysis EPMA Vacuum Systems What s the point? Key points Description of numbers of air molecules with different pumps ... – PowerPoint PPT presentation
Number of Views:49
Avg rating:3.0/5.0
Title: Vacuum Systems
1
UW- Madison Geology 777
Electron Probe MicroanalysisEPMA
- Vacuum Systems
2
Whats the point?
UW- Madison Geology 777
We need a high vacuum in the column and chamber
to maximize electron-sample interactions (not
electron-gas molecules). We need a high vacuum in
the gun to prolong the life of the electron
source and avoid arcing. We need automatic
microprocessor control and integration of vacuum
reading, venting, and gun and column settings to
avoid catastrophes.
3
Key points
UW- Madison Geology 777
- Description of numbers of air molecules with
different pumps - Rough vacuum pumps
- Diffusion and turbo pumps
- Ion pumps
- How vacuum is measured
4
Units of Vacuum
The two main units used to measure pressure
(vacuum) are torr and Pascal. Atmospheric
pressure (STD) 760 torr or 1.01x105 Pascal. One
torr 133.32 Pascal One Pascal 0.0075 torr An
excellent vacuum in the electron microprobe
chamber is 4x10-5 Pa (which is 3x10 -7 torr)
5
Why the fuss?
UW- Madison Geology 777
Anthony Buonaquisti wrote an excellent article
If you hate vacuum systems, read on published
in Microscopy Today. No one got involved in
electron microscopy in order to learn about
vacuum science. But the equipment we use responds
to poor vacuum practice with poor vacuum quality
-- which translates to equipment that doesnt
work well, or doesnt work at all. In the
following table, he demonstrates the magnitude of
the presence of gas molecules, which species
dominate at different pressure ranges, the vacuum
we achieve with different pumps, and the average
distance between molecules colliding with each
other (MFP).
6
Vacuum Regimes Whats what
UW- Madison Geology 777
7
Pumps
UW- Madison Geology 777
Electron microprobes (and SEMs and
TEMs) all have similiar pumping systems, being
combinations of at least 2 different pump types.
To go from atmospheric to moderate vacuum, rough
vacuum pumps are utilized. Once the chamber is
pumped to a level of 10-3 torr, high vacuum is
acheived via either a diffusion or turbo pump.
Some instruments (e.g. Cameca) include additional
(differential) pumping for the gun, via an ion
pump.
8
Rough vacuum pump
UW- Madison Geology 777
Gas molecules from the volume being pumped
diffuse into the space between the rotor and
chamber case, and are compressed by the rotating
rotor until they have a pressure high enough to
force upon the exhaust valve. They then exit,
through the oil, to the outlet port. Gas
molecules in the pressure range here (from atm
down to 10-2 - 10 -3 torr) move via viscous flow.
Oil-sealed rotary-vane rough vacuum pump
Rough vacuum pumps serve several functions to
rough out chambers vented to atmosphere, and
also to back higher vacuum pumps (e.g.
diffusion pumps).
Bigelow, Fig 4.1, p. 135
9
Oil Diffusion Pump
UW- Madison Geology 777
Bigelow suggests this well-known pump might be
better called a vapor jet pump. High molecular
mass oil is heated and moves vertically at
300-400 m/s, compressing against the jets any air
molecules that have diffused into the vicinity.
The oil molecules and now attached air molecules
fall downward, cooling to a liquid against the
water-cooled outer jacket. There is thus a build
up of air molecules in the lower region, adjacent
to the port that is attached to a second pump
(e.g. rotary-vane rough vacuum pump), which then
remove these air molecules.The pressures (to 10-7
torr) this pump operates at are appropriate for
the gas molecules to move by molecular flow (not
viscous flow) -- leading to backstreaming of oil
vapor (explained later)
Bigelow, Fig 5.1 2, p. 173
10
Molecular flow vs viscous flow
UW- Madison Geology 777
Initial pumping of volumes exposed to the
atmosphere proceed through the viscous flow
regime, where there are so many gas molecules
that their mean free path (MFP) is so short that
they collide more readily with each other than
with the walls of the tube. They move as a mass
in the general direction of low pressure.
When gas pressure drops enough that the MFP is
greater than the internal tube diameter,
individual gas molecules do not encounter other
gas molecules necessarily moving in one direction
(to low pressure). Rather, in this molecular flow
regime, the flow of
gas in independent of pressure gradient, and
depends mainly on tube dimensions and molecule
speed (temperature). In this case, backstreaming
of molecules into the high vacuum chamber is
possible.
Bigelow, Fig 2,1, p. 31
11
Backstreaming
UW- Madison Geology 777
Backstreaming refers to the movement of
gases (including pump oil vapor) from pumps into
the vacuum chamber. It can be an important issue
with diffusion pumps. Design of diffusion
pumps can make some difference. Placement of a
continuous operation cold plate over the
diffusion would be the best solution, but it is
rarely included in microprobe design.
Oil diffusion pumps have a long history and are
considered by many to be less costly and easier
to use in a multiple user facility. However, the
alternative is the turbo pump.
12
Turbo Pumps
UW- Madison Geology 777
Turbomolecular pumps use no oil (though
they may have greased bearings) and operate like
jet engines. Momentum is imparted to gas
molecules by disks rotating at very high speeds.
Gas molecules randomly entering the entrance
collide with the spinning rotor blade, and are
propelled toward the pumps exhaust vent. Turbo
pumps can reach 10-7 to 10 -10 torr. Turbo
pumps are nearly free of oil backstreaming (if
certain operating procedures are carefully
followed), as the high molecular mass oil vapor
is compressed to a ratio gt 1040 , versus values
of 1010 for nitrogen.
Bigelow, Fig 6.1, p. 229
13
SEM vacuum setup
UW- Madison Geology 777
This is a typical vacuum setup, with one high
vacuum pump (diffusion or turbo) and one rough
pump, and a series of valves. 1) Initial pump
down V1 and V2 closed, V3 open, and chamber,
manifold and gun pumped out. 2) When chamber
pressure low enough, V3 closes, V2 opens and
roughs out the diffusion/turbo pump. 3) When the
pressure in the diffusion/turbo pump is low
enough, V1 opens and pumps out the chamber and
gun to the high operating vacuum. Not shown is an
airlock chamber that would have its own vacuum
tubing and valves.
Bigelow, Fig 2,6, p. 42
14
Ion pump-1
UW- Madison Geology 777
Ion pumps are used for ultra high vacuums
they might also be called Getter pumps. They
consist of short stainless steel cylinders
(anodes) between two metal (Ti, or Ti and Ta)
plates (cathodes), all sitting within a strong
magnetic field parallel to the cylinder axes.
A high voltage is applied between the anodes
and cathodes, with the resulting electrons from
the cathodes moving in long helical trajectories
through the anode tubes, increasing the
probability of collision with gas molecules.
15
Ion pump-2
UW- Madison Geology 777
Pumping of gas molecules in an ion pump
occurs by 4 mechanisms 1) Surface burial
Ionized gas molecules then accelerate into the
cathode, sputtering Ti everywhere. Gas molecules
on surfaces are buried by Ti atoms. This is the
main pumping mechanism.
2) Chemisorption Reactive gases
(O, N, CO and H) react with fresh Ti to form
stable oxides, carbides, nitrides and hydrides.
3) Ion
implantation occurs when the electric potential
gives some positive gas ions sufficient energy to
penetrate the cathodes.
4) Neutral atom implantation some
gas ions strike the cathode and gain
electrons, becoming neutral atoms. If they
rebound with enough kinetic energy, they will
become buried in the surface of the anode or
opposite cathode. One problem with some
ion pumps is Argon instability, particularly if
there is a leak of P10 detector gas (90 Ar) into
the chamber.
Bigelow, Fig 7,1, p. 277
16
Measuring vacuum
UW- Madison Geology 777
torr
No one gauge can measure pressure from atmosphere
to UHV. Different gauges are used to measure
vacuum over different pressure segments. There
are 3 basic mechanisms
1) Mechanical use diaphragms
that change position due to force of the gas
molecules.
2) Gas property gauges measure a bulk property
such as thermal conductivity or viscosity.
3) Ionization gauges operate by
measuring the current flowing across ionized gas
molecules in the gauge.
Left image from Bigelow Right image from Physics
Today advertisement (MKS company)
17
Thermacouple Gauges
UW- Madison Geology 777
These gas property gauges find much usage
in our instruments. A thermocouple measures the
temperature of a heated wire inside a tube. As
the number of gas molecules hitting the wire (and
thus conducting heat away from the wire)
decreases as the pressure decreases, the
temperature of the wire increases. As temperature
rises, the voltage generated by the thermocouple
increases. This is calibrated and gives a precise
reading.
18
Ionization Gauges
UW- Madison Geology 777
Cold cathode ionization (aka Penning)
gauges do not have filaments, and rely on an
external event (cosmic ray, radioactive event) to
start the action. Once started, the magnetic
field constrains the electron to a long helical
path with a high probability of of ionizing gas
molecules. The current that flows across the gap
between anode and cathode is measured with a
sensitive microammeter calibrated in pressure
units (as less molecules ionized, the current is
lower).
19
RGA
UW- Madison Geology 777
Residual gas analyzers are specialized
mass spectrometers, used to detect and quantify
the gas partial pressures in a vacuum chamber.
They may be of the magnetic sector design, or
quadrupole design (above). Gas molecules are
ionized, and then accelerated into an ion
detector, separated by their mass-to-charge ratio
(m/z)
20
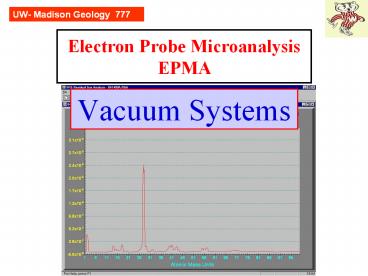
A snapshot of the vacuum in our SX51 chamber
UW- Madison Geology 777
Residual Gas Analyzers (RGAs) are rarely utilized
in electron microprobes. They are valuable for
diagnosing vacuum problems, as well as giving us
an appreciation that a vacuum is full of gas
molecules. The fact that the N (28) peak is 10x
the water (18) peak indicates that there is a
minor but significant leak of room air into the
chamber. Normally immediately after pumpdown, N
drops quickly with H2O
being the dominant gas, and then H2O slowly drops
too. From prior records of good vacuum, we can
deduce that the atm gases are at least one order
of magnitude too high.
21
References
UW- Madison Geology 777
Many vacuum product distributors publish catalogs
which include excellent descriptions of vacuum
system operations. One I find particularly
useful is Kurt. J. Lesker Company, and their
catalog is also online at www.lesker.com