Chapter 12 (Part a) Reactions of Arenes: Electrophilic Aromatic Substitution - PowerPoint PPT Presentation
1 / 150
Title:
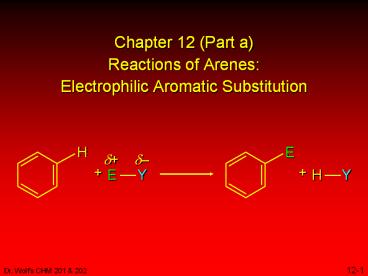
Chapter 12 (Part a) Reactions of Arenes: Electrophilic Aromatic Substitution
Description:
Title: Carey Chapter 12 Aromatic Sub. Author: Monte Wolf Last modified by: mwolf Created Date: 8/14/2000 8:05:35 PM Document presentation format: On-screen Show (4:3) – PowerPoint PPT presentation
Number of Views:575
Avg rating:3.0/5.0
Title: Chapter 12 (Part a) Reactions of Arenes: Electrophilic Aromatic Substitution
1
Chapter 12 (Part a)Reactions of
ArenesElectrophilic Aromatic Substitution
Dr. Wolf's CHM 201 202
12-1
2
Representative Electrophilic Aromatic
Substitution Reactions of Benzene
Dr. Wolf's CHM 201 202
12-2
3
Electrophilic aromatic substitutions include
- Nitration
- Sulfonation
- Halogenation
- Friedel-Crafts Alkylation
- Friedel-Crafts Acylation
Dr. Wolf's CHM 201 202
12-3
4
Table 12.1 Nitration of Benzene
H2SO4
HONO2
H2O
Nitrobenzene(95)
Dr. Wolf's CHM 201 202
12-4
5
Table 12.1 Sulfonation of Benzene
heat
HOSO2OH
H2O
Benzenesulfonic acid(100)
Dr. Wolf's CHM 201 202
12-5
6
Table 12.1 Halogenation of Benzene
FeBr3
Br2
HBr
Bromobenzene(65-75)
Dr. Wolf's CHM 201 202
12-6
7
Table 12.1 Friedel-Crafts Alkylation of Benzene
AlCl3
(CH3)3CCl
HCl
tert-Butylbenzene(60)
Dr. Wolf's CHM 201 202
12-7
8
Table 12.1 Friedel-Crafts Acylation of Benzene
AlCl3
HCl
1-Phenyl-1-propanone(88)
Dr. Wolf's CHM 201 202
12-8
9
Mechanistic PrinciplesofElectrophilic Aromatic
Substitution
Dr. Wolf's CHM 201 202
12-9
10
Step 1 attack of electrophileon p-electron
system of aromatic ring
- highly endothermic
- carbocation is allylic, but not aromatic
Dr. Wolf's CHM 201 202
12-10
11
Step 2 loss of a proton from the
carbocationintermediate
H
H
E
H
H
H
H
E
H
H
H
H
H
H
- highly exothermic
- this step restores aromaticity of ring
Dr. Wolf's CHM 201 202
12-11
12
Dr. Wolf's CHM 201 202
12-12
13
Based on this general mechanism
- what remains is to identify the electrophile in
nitration, sulfonation, halogenation,
Friedel-Crafts alkylation, and Friedel-Crafts
acylation to establish the mechanism of specific
electrophilic aromatic substitutions
Dr. Wolf's CHM 201 202
12-13
14
Nitration of Benzene
Dr. Wolf's CHM 201 202
12-14
15
Nitration of Benzene
H2SO4
HONO2
H2O
Electrophile isnitronium ion
Dr. Wolf's CHM 201 202
12-15
16
Step 1 attack of nitronium cationon p-electron
system of aromatic ring
Dr. Wolf's CHM 201 202
12-16
17
Step 2 loss of a proton from the
carbocationintermediate
H
H
NO2
H
H
H
H
NO2
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-17
18
Where does nitronium ion come from?
H2SO4
Dr. Wolf's CHM 201 202
12-18
19
Sulfonation of Benzene
Dr. Wolf's CHM 201 202
12-19
20
Sulfonation of Benzene
heat
HOSO2OH
H2O
Several electrophiles present a major one is
sulfur trioxide
Dr. Wolf's CHM 201 202
12-20
21
Step 1 attack of sulfur trioxideon p-electron
system of aromatic ring
Dr. Wolf's CHM 201 202
12-21
22
Step 2 loss of a proton from the
carbocationintermediate
H
H
SO3
H
H
H
H
SO3
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-22
23
Step 3 protonation of benzenesulfonate ion
H2SO4
Dr. Wolf's CHM 201 202
12-23
24
Halogenation of Benzene
Dr. Wolf's CHM 201 202
12-24
25
Halogenation of Benzene
FeBr3
Br2
HBr
Electrophile is a Lewis acid-Lewis basecomplex
between FeBr3 and Br2.
Dr. Wolf's CHM 201 202
12-25
26
The Br2-FeBr3 Complex
FeBr3
Lewis base
Lewis acid
Complex
- The Br2-FeBr3 complex is more electrophilic than
Br2 alone.
Dr. Wolf's CHM 201 202
12-26
27
Step 1 attack of Br2-FeBr3 complex on
p-electron system of aromatic ring
Br
Br
FeBr3
H
H
H
H
H
H
FeBr4
Dr. Wolf's CHM 201 202
12-27
28
Step 2 loss of a proton from the
carbocationintermediate
H
H
Br
H
H
H
H
Br
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-28
29
Friedel-Crafts Alkylation of Benzene
Dr. Wolf's CHM 201 202
12-29
30
Friedel-Crafts Alkylation of Benzene
AlCl3
(CH3)3CCl
HCl
Electrophile is tert-butyl cation
Dr. Wolf's CHM 201 202
12-30
31
Role of AlCl3
- acts as a Lewis acid to promote ionizationof the
alkyl halide
(CH3)3C
Cl
AlCl3
Dr. Wolf's CHM 201 202
12-31
32
Role of AlCl3
- acts as a Lewis acid to promote ionizationof the
alkyl halide
(CH3)3C
Cl
AlCl3
(CH3)3C
Dr. Wolf's CHM 201 202
12-32
33
Step 1 attack of tert-butyl cationon
p-electron system of aromatic ring
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-33
34
Step 2 loss of a proton from the
carbocationintermediate
H
H
C(CH3)3
H
H
H
H
C(CH3)3
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-34
35
Rearrangements in Friedel-Crafts Alkylation
- Carbocations are intermediates.
- Therefore, rearrangements can occur
(CH3)2CHCH2Cl
Isobutyl chloride
tert-Butylbenzene(66)
Dr. Wolf's CHM 201 202
12-35
36
Rearrangements in Friedel-Crafts Alkylation
- Isobutyl chloride is the alkyl halide.
- But tert-butyl cation is the electrophile.
(CH3)2CHCH2Cl
Isobutyl chloride
tert-Butylbenzene(66)
Dr. Wolf's CHM 201 202
12-36
37
Rearrangements in Friedel-Crafts Alkylation
H
H3C
C
CH2
CH3
Dr. Wolf's CHM 201 202
12-37
38
Reactions Related to Friedel-Crafts Alkylation
H2SO4
Cyclohexylbenzene(65-68)
- Cyclohexene is protonated by sulfuric acid,
giving cyclohexyl cation which attacks the
benzene ring
Dr. Wolf's CHM 201 202
12-38
39
Friedel-Crafts Acylation of Benzene
Dr. Wolf's CHM 201 202
12-39
40
Friedel-Crafts Acylation of Benzene
O
O
CCH2CH3
AlCl3
CH3CH2CCl
HCl
Electrophile is an acyl cation
Dr. Wolf's CHM 201 202
12-40
41
Step 1 attack of the acyl cationon p-electron
system of aromatic ring
H
H
H
H
H
H
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-41
42
Step 2 loss of a proton from the
carbocationintermediate
H
H
H
H
H
H
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-42
43
Acid Anhydrides
- can be used instead of acyl chlorides
AlCl3
Acetophenone(76-83)
Dr. Wolf's CHM 201 202
12-43
44
Acylation-Reduction
Dr. Wolf's CHM 201 202
12-44
45
Acylation-Reduction
permits primary alkyl groups to be attachedto an
aromatic ring
RCCl
AlCl3
Zn(Hg), HCl
CH2R
- Reduction of aldehyde and ketonecarbonyl groups
using Zn(Hg) and HCl is called the Clemmensen
reduction.
Dr. Wolf's CHM 201 202
12-45
46
Acylation-Reduction
permits primary alkyl groups to be attachedto an
aromatic ring
RCCl
H2NNH2, KOH,triethylene glycol,heat
AlCl3
- Reduction of aldehyde and ketonecarbonyl groups
by heating with H2NNH2 and KOH is called
theWolff-Kishner reduction.
CH2R
Dr. Wolf's CHM 201 202
12-46
47
Example Prepare isobutylbenzene
(CH3)2CHCH2Cl
CH2CH(CH3)2
AlCl3
- No! Friedel-Crafts alkylation of benzene using
isobutyl chloride fails because of rearrangement.
Dr. Wolf's CHM 201 202
12-47
48
Recall
(CH3)2CHCH2Cl
Isobutyl chloride
tert-Butylbenzene(66)
Dr. Wolf's CHM 201 202
12-48
49
Use Acylation-Reduction Instead
AlCl3
Zn(Hg)HCl
Dr. Wolf's CHM 201 202
12-49
50
Rate and Regioselectivity in Electrophilic
Aromatic Substitution
- A substituent already present on the ring can
affect both the rate and regioselectivityof
electrophilic aromatic substitution.
Dr. Wolf's CHM 201 202
12-50
51
Effect on Rate
- Activating substituents increase the rate of
EAS compared to that of benzene. - Deactivating substituents decrease the rate of
EAS compared to benzene.
Dr. Wolf's CHM 201 202
12-51
52
Methyl Group
- Toluene undergoes nitration 20-25 times faster
than benzene. - A methyl group is an activating substituent.
Dr. Wolf's CHM 201 202
12-52
53
Trifluoromethyl Group
- (Trifluoromethyl)benzene undergoes nitration
40,000 times more slowly than benzene . - A trifluoromethyl group is adeactivating
substituent.
Dr. Wolf's CHM 201 202
12-53
54
Effect on Regioselectivity
- Ortho-para directors direct an incoming
electrophile to positions ortho and/or para to
themselves. - Meta directors direct an incoming electrophile
to positions meta to themselves.
Dr. Wolf's CHM 201 202
12-54
55
Nitration of Toluene
34
3
63
- o- and p-nitrotoluene together comprise 97 of
the product - a methyl group is an ortho-para director
Dr. Wolf's CHM 201 202
12-55
56
Nitration of (Trifluoromethyl)benzene
3
91
6
- m-nitro(trifluoromethyl)benzene comprises 91 of
the product - a trifluoromethyl group is a meta director
Dr. Wolf's CHM 201 202
12-56
57
Rate and Regioselectivityin theNitration of
Toluene
Dr. Wolf's CHM 201 202
12-57
58
Carbocation Stability Controls Regioselectivity
gives ortho
gives para
gives meta
Dr. Wolf's CHM 201 202
12-58
59
Carbocation Stability Controls Regioselectivity
gives ortho
gives para
gives meta
more stable
less stable
Dr. Wolf's CHM 201 202
12-59
60
ortho Nitration of Toluene
CH3
NO2
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-60
61
ortho Nitration of Toluene
CH3
CH3
NO2
NO2
H
H
H
H
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-61
62
ortho Nitration of Toluene
CH3
CH3
CH3
NO2
NO2
NO2
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
- this resonance form is a tertiary carbocation
Dr. Wolf's CHM 201 202
12-62
63
ortho Nitration of Toluene
CH3
CH3
CH3
NO2
NO2
NO2
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
- the rate-determining intermediate in the
orthonitration of toluene has tertiary
carbocation character
Dr. Wolf's CHM 201 202
12-63
64
para Nitration of Toluene
Dr. Wolf's CHM 201 202
12-64
65
para Nitration of Toluene
- this resonance form is a tertiary carbocation
Dr. Wolf's CHM 201 202
12-65
66
para Nitration of Toluene
- this resonance form is a tertiary carbocation
Dr. Wolf's CHM 201 202
12-66
67
para Nitration of Toluene
- the rate-determining intermediate in the
paranitration of toluene has tertiary
carbocation character
Dr. Wolf's CHM 201 202
12-67
68
meta Nitration of Toluene
Dr. Wolf's CHM 201 202
12-68
69
meta Nitration of Toluene
Dr. Wolf's CHM 201 202
12-69
70
meta Nitration of Toluene
- all the resonance forms of the rate-determining
intermediate in the meta nitration of toluene
have their positive charge on a secondary carbon
Dr. Wolf's CHM 201 202
12-70
71
Nitration of Toluene Interpretation
- The rate-determining intermediates for ortho and
para nitration each have a resonance form that is
a tertiary carbocation. All of the resonance
forms for the rate-determining intermediate in
meta nitration are secondary carbocations. - Tertiary carbocations, being more stable, are
formed faster than secondary ones. Therefore,
the intermediates for attack at the ortho and
para positions are formed faster than the
intermediate for attack at the meta position.
This explains why the major products are o- and
p-nitrotoluene.
Dr. Wolf's CHM 201 202
12-71
72
Nitration of Toluene Partial Rate Factors
- The experimentally determined reaction rate can
be combined with the ortho/meta/para distribution
to give partial rate factors for substitution at
the various ring positions. - Expressed as a numerical value, a partial rate
factor tells you by how much the rate of
substitution at a particular position is faster
(or slower) than at a single position of benzene.
Dr. Wolf's CHM 201 202
12-72
73
Nitration of Toluene Partial Rate Factors
1
42
42
1
1
2.5
2.5
1
1
1
58
- All of the available ring positions in toluene
are more reactive than a single position of
benzene. - A methyl group activates all of the ring
positions but the effect is greatest at the ortho
and para positons. - Steric hindrance by the methyl group makes each
ortho position slightly less reactive than para.
Dr. Wolf's CHM 201 202
12-73
74
Nitration of Toluene vs. tert-Butylbenzene
- tert-Butyl is activating and ortho-para
directing - tert-Butyl crowds the ortho positions and
decreases the rate of attack at those positions.
Dr. Wolf's CHM 201 202
12-74
75
Generalization
- all alkyl groups are activating and ortho-para
directing
Dr. Wolf's CHM 201 202
12-75
76
Theory of Directing Effects
77
Rate and Regioselectivityin theNitration of
(Trifluoromethyl)benzene
Dr. Wolf's CHM 201 202
12-76
78
A Key Point
- A methyl group is electron-donating and
stabilizes a carbocation. - Because F is so electronegative, a CF3 group
destabilizes a carbocation.
Dr. Wolf's CHM 201 202
12-77
79
Carbocation Stability Controls Regioselectivity
gives ortho
gives para
gives meta
Dr. Wolf's CHM 201 202
12-78
80
Carbocation Stability Controls Regioselectivity
gives ortho
gives para
gives meta
less stable
more stable
Dr. Wolf's CHM 201 202
12-79
81
ortho Nitration of (Trifluoromethyl)benzene
CF3
NO2
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-80
82
ortho Nitration of (Trifluoromethyl)benzene
CF3
CF3
NO2
NO2
H
H
H
H
H
H
H
H
H
H
Dr. Wolf's CHM 201 202
12-81
83
ortho Nitration of (Trifluoromethyl)benzene
CF3
CF3
CF3
NO2
NO2
NO2
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
- this resonance form is destabilized
Dr. Wolf's CHM 201 202
12-82
84
ortho Nitration of (Trifluoromethyl)benzene
CF3
CF3
CF3
NO2
NO2
NO2
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
- one of the resonance forms of the
rate-determining intermediate in the
orthonitration of (trifluoromethyl)benzene is
strongly destabilized
Dr. Wolf's CHM 201 202
12-83
85
para Nitration of (Trifluoromethyl)benzene
Dr. Wolf's CHM 201 202
12-84
86
para Nitration of (Trifluoromethyl)benzene
- this resonance form is destabilized
Dr. Wolf's CHM 201 202
12-85
87
para Nitration of (Trifluoromethyl)benzene
- this resonance form is destabilized
Dr. Wolf's CHM 201 202
12-86
88
para Nitration of (Trifluoromethyl)benzene
- one of the resonance forms of the
rate-determining intermediate in the
paranitration of (trifluoromethyl)benzene is
strongly destabilized
Dr. Wolf's CHM 201 202
12-87
89
meta Nitration of (Trifluoromethyl)benzene
Dr. Wolf's CHM 201 202
12-88
90
meta Nitration of (Trifluoromethyl)benzene
Dr. Wolf's CHM 201 202
12-89
91
meta Nitration of (Trifluoromethyl)benzene
- none of the resonance forms of the
rate-determining intermediate in the meta
nitration of (trifluoromethyl)benzene have their
positive charge on the carbon that bears the CF3
group
Dr. Wolf's CHM 201 202
12-90
92
Nitration of (Trifluoromethyl)benzene
Interpretation
- The rate-determining intermediates for ortho and
para nitration each have a resonance form in
which the positive charge is on a carbon that
bears a CF3 group. Such a resonance structure is
strongly destabilized. The intermediate in meta
nitration avoids such a structure. It is the
least unstable of three unstable intermediates
and is the one from which most of the product is
formed.
Dr. Wolf's CHM 201 202
12-91
93
Nitration of (Trifluoromethyl)benzenePartial
Rate Factors
- All of the available ring positions in
(trifluoromethyl)benzene are much less reactive
than a single position of benzene. - A CF3 group deactivates all of the ring
positions but the degree of deactivation is
greatest at the ortho and para positons.
Dr. Wolf's CHM 201 202
12-92
94
Theory of Directing Effects
95
Substituent Effects in ElectrophilicAromatic
SubstitutionActivating Substituents
Dr. Wolf's CHM 201 202
12-93
96
Table 12.2
Classification of Substituents in Electrophilic
Aromatic Substitution Reactions
- Very strongly activating
- Strongly activating
- Activating
- Standard of comparison is H
- Deactivating
- Strongly deactivating
- Very strongly deactivating
Dr. Wolf's CHM 201 202
12-94
97
Generalizations
- 1. All activating substituents are ortho-para
directors. - 2. Halogen substituents are slightly
deactivating but ortho-para directing. - 3. Strongly deactivating substituents are meta
directors.
Dr. Wolf's CHM 201 202
12-95
98
Electron-Releasing Groups (ERGs)
- are ortho-para directing and activating
ERG
ERGs include R, Ar, and CC
Dr. Wolf's CHM 201 202
12-96
99
Electron-Releasing Groups (ERGs)
- are ortho-para directing and strongly activating
ERG
ERGs such as OH, and OR arestrongly activating
Dr. Wolf's CHM 201 202
12-97
100
Nitration of Phenol
- occurs about 1000 times faster than nitration of
benzene
HNO3
44
56
Dr. Wolf's CHM 201 202
12-98
101
Bromination of Anisole
- FeBr3 catalyst not necessary
Br2
aceticacid
90
Dr. Wolf's CHM 201 202
12-99
102
Oxygen Lone Pair Stabilizes Intermediate
H
H
H
H
Br
H
- all atomshave octets
Dr. Wolf's CHM 201 202
12-100
103
Electron-Releasing Groups (ERGs)
ERG
- ERGs with a lone pair on the atom
directlyattached to the ring are ortho-para
directingand strongly activating
Dr. Wolf's CHM 201 202
12-101
104
Examples
- All of these are ortho-para directingand
strongly to very strongly activating
Dr. Wolf's CHM 201 202
12-102
105
Lone Pair Stabilizes Intermediates forortho and
para Substitution
- comparable stabilization not possible for
intermediate leading to meta substitution
Dr. Wolf's CHM 201 202
12-103
106
Substituent Effects in ElectrophilicAromatic
SubstitutionStrongly Deactivating Substituents
Dr. Wolf's CHM 201 202
12-104
107
ERGs Stabilize Intermediates forortho and para
Substitution
Dr. Wolf's CHM 201 202
12-105
108
Electron-withdrawing Groups (EWGs)
DestabilizeIntermediates for ortho and para
Substitution
EWG
EWG
X
H
H
H
H
H
H
H
H
X
H
H
- CF3 is a powerful EWG. It is strongly
deactivating and meta directing
Dr. Wolf's CHM 201 202
12-106
109
Many EWGs Have a Carbonyl GroupAttached Directly
to the Ring
EWG
- All of these are meta directing and strongly
deactivating
Dr. Wolf's CHM 201 202
12-107
110
Other EWGs Include
EWG
NO2
SO3H
- All of these are meta directing and strongly
deactivating
Dr. Wolf's CHM 201 202
12-108
111
Nitration of Benzaldehyde
HNO3
H2SO4
75-84
Dr. Wolf's CHM 201 202
12-109
112
Problem 12.14(a) page 468
Cl
Cl2
FeCl3
62
Dr. Wolf's CHM 201 202
12-110
113
Disulfonation of Benzene
HO3S
SO3
SO3H
H2SO4
90
Dr. Wolf's CHM 201 202
12-111
114
Bromination of Nitrobenzene
Br
Br2
NO2
NO2
Fe
60-75
Dr. Wolf's CHM 201 202
12-112
115
Substituent Effects in ElectrophilicAromatic
SubstitutionHalogens
- F, Cl, Br, and I are ortho-para directing,but
deactivating
Dr. Wolf's CHM 201 202
12-113
116
Nitration of Chlorobenzene
HNO3
H2SO4
69
1
30
- The rate of nitration of chlorobenzene is about
30 times slower than that of benzene.
Dr. Wolf's CHM 201 202
12-114
117
Nitration of Toluene vs. Chlorobenzene
Cl
0.029
0.029
0.009
0.009
0.137
Dr. Wolf's CHM 201 202
12-115
118
Halogens
- thus, for the halogens, the inductive and
resonance effects run counter to each other, but
the former is somewhat stronger - the net effect is that halogens are deactivating
but ortho-para directing
119
Multiple Substituent Effects
Dr. Wolf's CHM 201 202
12-116
120
The Simplest Case
- all possible EAS sites may be equivalent
CH3
CCH3
AlCl3
CH3
99
Dr. Wolf's CHM 201 202
12-117
121
Another Straightforward Case
CH3
Br
NO2
86-90
- directing effects of substituents reinforceeach
other substitution takes place orthoto the
methyl group and meta to the nitro group
Dr. Wolf's CHM 201 202
12-118
122
Generalization
- regioselectivity is controlled by themost
activating substituent
Dr. Wolf's CHM 201 202
12-119
123
The Simplest Case
- all possible EAS sites may not be equivalent
strongly activating
Br2
aceticacid
87
Dr. Wolf's CHM 201 202
12-120
124
When activating effects are similar...
CH3
NO2
C(CH3)3
88
- substitution occurs ortho to the smaller group
Dr. Wolf's CHM 201 202
12-121
125
Steric effects control regioselectivity
whenelectronic effects are similar
98
- position between two substituents is
lastposition to be substituted
Dr. Wolf's CHM 201 202
12-122
126
Regioselective Synthesis of Disubstituted
Aromatic Compounds
Dr. Wolf's CHM 201 202
12-123
127
Factors to Consider
- order of introduction of substituents to ensure
correct orientation
Dr. Wolf's CHM 201 202
12-124
128
Synthesis of m-Bromoacetophenone
- Which substituent should be introduced first?
Dr. Wolf's CHM 201 202
12-125
129
Synthesis of m-Bromoacetophenone
para
- If bromine is introduced first,
p-bromoacetophenone is major product.
meta
Dr. Wolf's CHM 201 202
12-126
130
Synthesis of m-Bromoacetophenone
Br2
AlCl3
AlCl3
Dr. Wolf's CHM 201 202
12-127
131
Factors to Consider
- order of introduction of substituents to ensure
correct orientation - Friedel-Crafts reactions (alkylation, acylation)
cannot be carried out on strongly deactivated
aromatics
Dr. Wolf's CHM 201 202
12-128
132
Synthesis of m-Nitroacetophenone
- Which substituent should be introduced first?
Dr. Wolf's CHM 201 202
12-129
133
Synthesis of m-Nitroacetophenone
- If NO2 is introduced first, the next step
(Friedel-Crafts acylation) fails.
Dr. Wolf's CHM 201 202
12-130
134
Synthesis of m-Nitroacetophenone
O2N
HNO3
H2SO4
AlCl3
Dr. Wolf's CHM 201 202
12-131
135
Factors to Consider
- order of introduction of substituents to ensure
correct orientation - Friedel-Crafts reactions (alkylation, acylation)
cannot be carried out on strongly deactivated
aromatics - sometimes electrophilic aromatic substitution
must be combined with a functional group
transformation
Dr. Wolf's CHM 201 202
12-132
136
Synthesis of p-Nitrobenzoic Acid from Toluene
- Which first? (oxidation of methyl group or
nitration of ring)
Dr. Wolf's CHM 201 202
12-133
137
Synthesis of p-Nitrobenzoic Acid from Toluene
nitration givesm-nitrobenzoicacid
oxidation givesp-nitrobenzoicacid
Dr. Wolf's CHM 201 202
12-134
138
Synthesis of p-Nitrobenzoic Acid from Toluene
HNO3
Na2Cr2O7, H2O H2SO4, heat
H2SO4
Dr. Wolf's CHM 201 202
12-135
139
Substitution in Naphthalene
Dr. Wolf's CHM 201 202
12-136
140
Naphthalene
H
H
1
H
H
2
H
H
H
H
- two sites possible for electrophilicaromatic
substitution - all other sites at which substitution can
occurare equivalent to 1 and 2
Dr. Wolf's CHM 201 202
12-137
141
EAS in Naphthalene
AlCl3
90
- is faster at C-1 than at C-2
Dr. Wolf's CHM 201 202
12-138
142
EAS in Naphthalene
E
E
H
H
- when attack is at C-1
- carbocation is stabilized by allylic resonance
- benzenoid character of other ring is maintained
Dr. Wolf's CHM 201 202
12-139
143
EAS in Naphthalene
E
H
- when attack is at C-2
- in order for carbocation to be stabilized by
allylic resonance, the benzenoid character of the
other ring is sacrificed
Dr. Wolf's CHM 201 202
12-140
144
Substitution inHeterocyclic Aromatic Compounds
Dr. Wolf's CHM 201 202
12-141
145
Generalization
- There is none.
- There are so many different kinds of
heterocyclicaromatic compounds that no
generalizationis possible. - Some heterocyclic aromatic compoundsare very
reactive toward electrophilicaromatic
substitution, others are very unreactive..
Dr. Wolf's CHM 201 202
12-142
146
Pyridine
- Pyridine is very unreactive it
resemblesnitrobenzene in its reactivity. - Presence of electronegative atom (N) in
ringcauses p electrons to be held more strongly
thanin benzene.
Dr. Wolf's CHM 201 202
12-143
147
Pyridine
SO3, H2SO4
HgSO4, 230C
71
- Pyridine can be sulfonated at high temperature.
- EAS takes place at C-3.
Dr. Wolf's CHM 201 202
12-144
148
Pyrrole, Furan, and Thiophene
- Have 1 less ring atom than benzene or pyridine
to hold same number of p electrons (6). - p electrons are held less strongly.
- These compounds are relatively reactive toward
EAS..
Dr. Wolf's CHM 201 202
12-145
149
Example Furan
BF3
CCH3
O
O
75-92
- undergoes EAS readilyC-2 is most reactive
position
Dr. Wolf's CHM 201 202
12-146
150
End of Chapter 12 (Part a)