Chapter 6 Ionic Reactions---Nucleophilic substitution and elimination reactions of alkylhalides (???????????????) - PowerPoint PPT Presentation
1 / 83
Title:
Chapter 6 Ionic Reactions---Nucleophilic substitution and elimination reactions of alkylhalides (???????????????)
Description:
Chapter 6 Ionic Reactions---Nucleophilic substitution and elimination reactions of alkylhalides ( – PowerPoint PPT presentation
Number of Views:352
Avg rating:3.0/5.0
Title: Chapter 6 Ionic Reactions---Nucleophilic substitution and elimination reactions of alkylhalides (???????????????)
1
Chapter 6 Ionic Reactions---Nucleophilic
substitution and elimination reactions of
alkylhalides (???????????????)
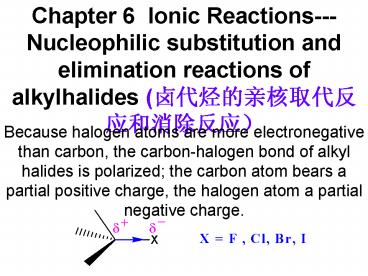
- Because halogen atoms are more electronegative
than carbon, the carbon-halogen bond of alkyl
halides is polarized the carbon atom bears a
partial positive charge, the halogen atom a
partial negative charge.
2
Table 6.1 Carbon-halogen bond lengths
3
Vinyl halides (????) or phenyl halides (????)
4
6.2 Physical properties of organic halides
- Very low solubilities in water
- They are miscible with each other and with other
relatively nonpolar solvents - CH2Cl, CHCl3 and CCl4 are often used as solvents
for nonpolar - CHCl3 and CCl4 have a cumulative toxicity and are
carcinogenic. - Polyfluoroalkanes have low boiling points,
- (Hexafluoroethane boils at 79o)
(???(?)?)
5
6.3 Reaction Mechanisms
- Mechanism of the reactionThe events that are
postulated to take place at the molecular level
as reactants become products - If the reaction takes place in more than one
step, then what are these steps, and what kinds
of intermediates intervene between reactants and
products?
6
6.3A Homolysis and heterolysis of covalent bonds
(?????????)
Covent bond may break in three possible ways
7
6.3B Reactive intermediates in organic chemistry
- Organic reactions that take place in more than
one step involve the formation of an
intermediate----one that results from either
homolysis or heterolysis of a bond. Homolysis of
a bond to carbon leads to an intermediate known
as a carbon radical (free radical)
8
Heterolysis of a bond can lead either to a
trivalent carbon cation or carbon anion
9
6.3C Ionic reactions (????) and radical
reactions(?????or ?????)
- In ionic reactions the bonds of the reacting
molecules undergo heterolysis - In radical reactions, they undergo homolysis (in
detail in chapter 7) - In this chapter we concern ourselves only with
ionic reactions.
10
6.4 Nucleophilic substitution reactions (??????)
11
Nucleophilic substitution reactions(??????)
- The carbon-halogen bond of the substrate
undergoes heterolysis, and the unshared pair of
the nucleophile is used to form a new bond to the
carbon atom
12
(No Transcript)
13
6.5 Nucleophiles njukliefailn.?????
- A nucleophile is a reagent that seeks a positive
center
14
Specific Example
15
6.5 A Leaving groups (????)
- To be a good leaving group the substituent must
be able to leave as a relatively stable, weakly
basic molecule or ion
16
6.7 Kinetics of a nucleophilic substitution
reaction An SN2 reaction
17
6.8 A mechanism for the SN2 reaction(SN2?????)
18
SN2 Reaction
???????SN2????.
19
6.9 Transition state theory Free-energy
diagrams (SN2)
20
Fig 6.6 A potential energy diagram for the
reaction of methyl chloride with hydroxide ion at
60 oC
21
Fig 6.3 A free-energy diagram for a
hypothetical reaction with a positive free-
energy change
22
6.10 The stereochemistry of SN2 reactions In SN2
reaction the nucleophile attacks from the
backside, that is, from the side directly
opposite the leaving group. This mode of attack
causes a change in the configuration of the
carbon atom that is the object of nucleophilic
attack
23
SN2 reaction------a configuration inversion
24
SN2 reactions always lead to inversion of
configuration
25
6.11 The reaction of tert-butyl chloride with
hydroxide ion An SN1 reaction
26
6.12 A mechanism for the SN1 reaction (multistep
reactions)
27
Fig 6.8 A free-energy diagram for the SN1
reaction of tert-butyl chloride with water
28
The important transition state for the SN1
reaction is the transition state of the
rate-determining step. In it the carbon-chlorine
bond of tert-butyl chloride is largely broken and
ions are beginning to develop. The solvent
(water) stabilizes these developing ions by
solvation
29
6.13 Carbocations (????)
6.13A The structure of carbocations
The central carbon atom in a carbocation is
electron deficient it has only six electrons in
its outside energy level.
30
6.13B The relative stabilities of carbocations
Tertiary carbocations are the most stable,
secondary carbocations are the stable, and the
primary carbocations are not stable.
31
Fig 6.10 How a methyl group helps stabilize the
positive charge of a carbocation.
32
Because of sigama-p conjugating. As a result, the
delocalization of charge and the order of
stability of the carbocations as follows
33
6.14 The stereochemistry of SN1 reactions
34
6.14A Reactions that involve racemization
(????????)
35
6.14B Solvolysis (?????)
The SN1 reaction of an alkyl halide with water is
an example of solvolysis
Examples of Solvolysis
36
In the last example the solvent is formic acid
(??,?? HCOOH) and the following take place
37
6.15 Factors affecting the rates of SN1 and SN2
reactions(??SN1 and SN2 ???????)
- 1. The structure of the substrate
- 2. The concentration and reactivity of the
nucleophile (for bimolecular reactions only) - The effect of the solvent.
- The nature of the leaving group.
38
6.15A The effect of the structure of the
substarate (???????)
General order of reactivity in SN2 reaction
39
The important factor behind this order of
reactivity is a steric effect (?????SN2?????)
40
SN1 reaction. The primary factor that determines
the reactivity of organic substrates in an SN1
reaction is the relative stability of the
carbocation that is formed
41
SN1 reaction
42
An SN1 Mechanism
- For a methyl, primary, or secondary halide to
react by an SN1 mechanism it would have to ionize
to form a methyl, primary, or secondary
carbocation. These carbocations, however, are
much higher in energy than a tertiary
carbocation, and the transition states leading to
these carbocations are higher in energy. The
activation energy for an SN1 reaction of a simple
methyl, primary or secondary halide,
consequently, is so large (the reaction is so
slow) .
43
6.15B The effect of the concentration and
strength of the nucleophile(???????????)
- Since the nucleophile does not participate in the
rate-determining step of an SN1 reaction, the
rates of SN1 reactions are unaffected by either
the concentration or the identity of the
nucleophile. The rates of SN2 reactions, however,
depend on both the concentration and the identity
of the attacking nucleophile. We saw that how
increasing the concentration of the nucleophile
increases the rate of an SN2 reaction.
44
We describe nucleophiles as being strong or weak.
When we do this we are really describing their
relative reactives in SN2 reactions. A strong
nucleophile is one that reacts rapidly with a
given substrate. A weak nucleophile is one that
reacts slowly with the same substrate under the
same reaction conditions.
45
The relative strengths of nucleophiles can be
correlated with two structural features
- 1. A negatively charged nucleophile is always a
stronger nucleophile than its conjugate acid in a
SN2 reaction.
46
2. In a group of nucleophiles in which the
nucleophilic atom is the same, nucleophilicities
parallel basicities
This is also their order of basicity. An alkoxide
ion (RO-) is a slightly stronger base than a
hydroxide ion (HO-), a hydroxide ion is a much
stronger base than a carboxylate ion (RCOO-), and
so on
47
6.15C Solvent effects on SN2 reactions Polar
protic and aprotic solvents (????????????)
- Protic solvent-----has a hydrogen atom attached
to an atom of a strongly electronegative element
(oxygen). ( water, alcohol etc.) - Aprotic solvent------Aprotic solvents are those
solvents whose molecules do not have a hydrogen
atom that is attached to an atom of a strongly
electronegative element.(Benzene, the alkanes,
etc.)
48
Relative Nucleophilicity in protic Solvents
Why?
49
Because molecules of protic solvents can form
hydrogen bonds to nucleophiles in the following
way
50
Polar Aprotic Solvents (???????)
Aprotic solvents are those solvents whose
molecules do not have a hydrogen atom that is
attached to an atom of a strongly electronegative
element.
51
Problem 6.6 Classify the following solvents as
being protic or aprotic
- Formic acid HCOOH
- Acetone CH3COCH3
- Acetonitrile CH3CN
- Formamide HCONH2
- Sulfur dioxide SO2
- Ammonia NH3
- Trimethylamine N(CH3)2
- Ethylene glycol HOCH2CH2OH
52
Problem 6.7 Would you expect the reaction of
propyl bromide with sodium cyanide (NaCN), that
is, to occur faster in DMF or in ethanol? Explain
your answer.
53
Explain the reaction is an SN2 reaction. the
nucleophile (CN-) will be relatively unencumbered
(?????) by solvent molecules, therefore, it will
be more reactive than in ethanol.
54
6.15D Solvent effects on SN1 reactions. The
ionizing ability of the solvent
- Because of its ability to solvate cations and
anions so effectively, the use of a polar protic
solvent will greatly increase the rate of
ionization of an alkyl halide in any SN1 reaction.
55
SN1 reaction in polar protic solvent
Water is the most effective solvent for promoting
ionization, but most organic compounds do not
dissolve appreciably in water. They usually
dissolve, however, in alcohols, and quite often
mixed solvents (methanol-water) are used.
56
Table 6.5 Dielectric constants of common
solvents (?????????)
57
6.15E The nature of the leaving group (???????)
- The Best leaving groups are those that become the
most stable ions after they depart. Since most
leaving groups leave as a negative ion, the best
leaving groups are those ions that stabilize a
negative charge most effectively. Because weak
bases do this best, the best leaving groups are
weak bases.
58
In either an SN1 or SN2 reaction the leaving
group begins to acquire a negative charge as the
transition state is reached
59
An iodide ion is the best leaving group and a
fluoride ion is the poorest
60
Other weak bases that are good leaving groups
61
The trifluoromethanesulfonate ion (CF3SO3-,
commonyl called the triflate ion (???????)
- CF3SO3-
- Trifate ion (a super leaving group)
- ROH (OH- rarely act as leaving groups).
62
Very powerful bases such as hydride ions (H-) and
alkanide ions (R-) never act as leaving groups.
63
6.15F Summary SN1 versus SN2
64
6.16 Organic synthesis???? Functional group
transformations using SN2 reactions
- The process of making one compound from another
is called synthesis. SN2 reactions are highly
useful in organic synthesis because they enable
us to convert one functional group into
another--- a process that is called a
functionalgroup transformation or a functional
group interconversion(?????)
65
SN2 reactions in organic synthesis
66
Alkyl chlorides and bromides are also easily
converted to alkyl iodides by nucleophilic
substitution reactions.
67
If we have available -2-bromobutane, we can
carry out the following synthesis
68
Problem 6.11 Starting with ( S)-2-brombutane,
outline syntheses of each of the following
compounds
69
Answer (a)
70
6.16A The unreactivity of vinylic (???) and
phenyl halides(???)
- A halogen atom attached to one carbon atom of a
double bond are called vinylic halides those
that have a halogen atom attached to a benzene
ring are called phenyl halides
71
6.17 Elimination reactions of alkylhalides
(????????))
- Another characteristic of alkyl halides is that
they undergo elimination reactions. In an
elimination reaction the fragments of some
molecule (YZ) are removed (eliminated) from
adjacent atoms of the reactant. This elimination
leads to the introduction of a multiple bond (??)
72
6.17A Dehydrohalogenation (????)
- A widely used method for synthesizing alkenes is
the elimination of HX from adjacent atoms of an
alkyl halide. Heating the alkyl halide with a
strong base causes the reaction to take place.
73
Dehydrohalogenation (????)
74
6.17B Bases used in dehydrohalogenation
- Various strong bases have been used for
dehydrohalogenations.
75
6.17C Mechanisms of Dehydrohalogenations
(??????)
- One mechanism is a bimolecular mechanism called
the E2 reaction the other is a unimolecular
mechanism called the E1 reaction
76
6.18 The E2 reaction
77
The E2 reaction
78
6.19 The E1 reaction
79
The E1 reaction mechanism
80
6.20 Substitution (SN1 and SN2) versus
Elimination (E2 and E1)
- Since eliminations occur best by an E2 path when
carried out with a high concentration of a strong
base ( and thus a high concentration of a strong
nucleophile), substitution reactions by an SN2
path often compete with the elimination reaction.
When the nucleophile attacks the carbon atom
bearing the leaving group, substitution results. - SN2 compete with E2, SN1 compete with E1.
81
The SN2 compete with E2
82
(No Transcript)
83
Sample problem In each case give the mechanism
(SN1, SN2, E1, or E2)