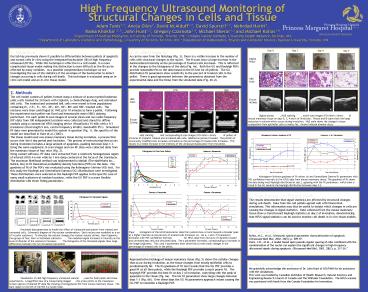
UHNResDay_Poster_2003 - PowerPoint PPT Presentation
Title:
UHNResDay_Poster_2003
Description:
High Frequency Ultrasound Monitoring of Structural Changes in Cells and Tissue Adam Tunis1,2, Anoja Giles2, David McAlduff1,2, David Spurrell1,2, Mehrdad Hariri2, – PowerPoint PPT presentation
Number of Views:29
Avg rating:3.0/5.0
Title: UHNResDay_Poster_2003
1
High Frequency Ultrasound Monitoring of
Structural Changes in Cells and Tissue Adam
Tunis1,2, Anoja Giles2, David McAlduff1,2, David
Spurrell1,2, Mehrdad Hariri2, Rama Khokha1,2,3,
John Hunt1,2, Gregory Czarnota2,4, Michael
Sherar1,2 and Michael Kolios1,4 1 Department of
Medical Biophysics, University of Toronto,
Toronto, ON 2 Ontario Cancer Institute,
University Health Network, Toronto, ON 3
Department of Laboratory Medicine and
Pathobiology, University of Toronto, Toronto, ON
4 Department of Mathematics, Physics and Computer
Science, Ryerson University, Toronto, ON
1. Objective Our lab has previously shown it
possible to differentiate between pellets of
apoptotic and normal cells in-vitro using the
integrated backscatter (IB) of high frequency
ultrasound (HFUS). While this technique is
effective in a cell model, in a more complicated
tissue model making this distinction is more
difficult as the IB can be affected by many
variables. As a possible complementary technique
we are investigating the use of the statistics of
the envelope of the backscatter to detect changes
occurring in cells during cell death. This
technique is evaluated using an in-vitro cell
model and an in-vivo tissue model.
3. In-Vitro Results As can be seen from the
histology (Fig. 3), there is a visible increase
in the number of cells with structural changes to
the nuclei. The B-scans show a large increase in
the backscattered intensity as the percentage of
treated cells increases. This is reflected in
the changes to the histograms of the data (Fig.
4a). Both the GG and Rayleigh PDFs provide
reasonable fits to the data based on the KS test
for all pellets. The GG distribution fit
parameters show sensitivity to the percent of
treated cells in the pellet. There is good
agreement between the parameters obtained from
the experimental data and the those from the
simulated data (Fig. 4b-d).
Figure 5 Digital photos (a-d), HE staining
(e-h), and B-scan images (FOV 8mm x 8mm) (i-l) of
mouse mammary tissue on days 0, 3, 4 and 6 of
involution. Photos and B-scans show the large
reduction in volume which occurs during
involution. HE stain shows the change in tissue
composition from epithelial cells to mostly fat.
Arrows indicate alveolar ducts.
Day 0
Day 4
Day 3
Day 6
Digital Photo
a)
b)
c)
d)
HE Stain
e)
g)
f)
h)
0
2.5
100
20
2. Methods The cell model consists of pellets
formed using a mixture of acute myeloid leukemia
(AML) cells treated for 24 hours with Cisplatin,
a chemotherapy drug, and untreated AML cells.
The treated and untreated AML cells were mixed to
form populations containing 0, 2.5, 5, 10,
20, 40, 60, 80 and 100 treated cells. The
mixtures were then centrifuged at 1942 g for 10
minutes to form a pellet. Following the
experiment each pellet was fixed and
hematoxylin-eosin (HE) staining performed. For
each pellet B-scan images of several slices and
raw radio frequency (RF) data from 100
independent locations were collected and stored
for offline analysis using a commercial HFUS
imaging device (VisualSonics VS-40b) with a f/3
transducer (focal length 9 mm, centre frequency
40 MHz, bandwidth 95). Simulated RF data were
generated to model the system in question (Fig.
1), the specifics of the model are described in
Hunt et al. (2002). The tissue model used was
mouse mammary tissue during involution, a process
that occurs over the 6 day period post-lactation.
The process of restructuring that occurs during
involution includes a large amount of apoptosis,
peaking between days 1-3. Using the same
equipment, B-scan images and raw RF data were
collected daily from the mammary tissue of four
mice (Fig. 2). Using custom software, RF data
were extracted from a relatively homogeneous
region of interest (ROI) 4-6 mm wide by 1 mm deep
centered at the focus of the transducer. The
maximum likelihood method was implemented in
Matlab (The MathWorks Inc., Natick, MA) to fit
theoretical probability density functions (PDFs)
to the data. The goodness of fit of the PDFs was
evaluated using the Kolmogorov-Smirnov (KS) test.
For this study the Rayleigh and Generalized
Gamma (GG) distributions were investigated.
These distributions were selected as the Rayleigh
PDF applies to the specific case of many small
scatterers at random locations, while the GG PDF
is a more flexible distribution with three
fitting parameters.
Figure 3 HE staining (a-d) and corresponding
B-scan images (FOV 8mm x 8mm) (e-h) of pellets of
mixtures of Cisplatin treated and untreated AML
cells, labelled as percent treated. The portion
of structurally modified cells increases
noticeably as the percentage of treated cells
increases. This results in a visible increase in
the intensity of the ultrasound backscatter from
the pellet.
HE Stain
B-scan 40 MHz
a)
b)
c)
d)
l)
j)
i)
k)
B-scan 20 MHz
h)
e)
f)
g)
a)
b)
Figure 6 Kolmogorov-Smirnov goodness of fit
values (a) and Generalized Gamma fit parameters
with 95 confidence intervals (b) for HFUS data
from mouse mammary tissue. The goodness of fit
shows the data being most Rayleigh-distributed at
day 2, agreeing with the fit parameters which
show a trend in the GG towards the Rayleigh
distribution between days 2-3.
Kolmogorov-Smirnov Goodness of Fit
Gamma a, c v Parameters
- Gamma a - Gamma v
- Gamma c
- Rayleigh - Gen. Gamma - Significance Level
b)
a)
Figure 4 Histograms of the HFUS backscatter data
from pellets show a trend towards a broader peak
at a higher intensity as the percent of treated
cells increases (a). GG a, c and v fit parameter
estimates with 95 confidence intervals (b, c
d) for HFUS data from mixtures of Cisplatin
treated and untreated AML cells and simulated
data. The a parameter increases, corresponding
to increases in the image brightness. The c and
v parameters show sensitivity to even small
changes in the percentage of treated cells in the
pellet.
Gamma a Parameter
Histograms for Data from Pellets
KS Value A.U.
Gamma a v A.U.
Gamma c A.U.
- Pellet
- Simulation
- 0 - 2.5 - 20 - 40 - 60 - 100
Gamma a (simulation) A.U.
Number of Counts A.U.
Gamma a (pellet) A.U.
Day of Involution
Day of Involution
Percent Treated
Intensity A.U.
c)
d)
Gamma c Parameter
Gamma v Parameter
5. Conclusions and Future Work The results
demonstrate that signal statistics are affected
by structural changes during cell death. Data
from the cell pellets agreed well with
theoretical simulations. This information may
thus be useful to isolate which changes in cells
are causing the changes in signal statistics.
Data collected from the mouse mammary tissue show
a trend toward Rayleigh statistics at day 2 of
involution, demonstrating that HFUS signal
statistics can be used to monitor cell death in
in-vivo tissue models.
Figure 1 Simulated data generated to model the
effect of ultrasound backscatter from treated and
untreated cells. Schematic diagram of the
nuclear condensation. Each nucleus was modelled
as a set of 16 point scatterers. To simulate the
cellular changes the nuclear volume shrinks, then
fragments into groups of four, then 16 individual
scatterers (a). The modelled signal increases in
intensity as the level of disorder of the
scatterers increases (b). The histograms of the
simulated signals show large differences between
the two simulated populations (c).
a)
Histograms for Simulated Signals
c)
- Pellet - Simulation
- Treated - Untreated
Gamma v A.U.
Gamma c A.U.
Number of Counts A.U.
- Pellet - Simulation
b)
Intensity A.U.
6. References Kolios, M.C., et al., Ultrasonic
spectral parameter characterization of apoptosis.
Ultrasound Med Biol, 2002. 28(5) p.
589-97. Hunt, J.W., et al., A model based upon
pseudo regular spacing of cells combined with the
randomisation of the nuclei can explain the
significant changes in high-frequency ultrasound
signals during apoptosis. Ultrasound Med Biol,
2002. 28(2) p. 217-26.
Percent Treated
Percent Treated
Figure 2 VisualSonics VS-40b high frequency
ultrasound scanner (a), used for both pellet and
tissue experiments. A magnified view of a mouse
being imaged (b), the arrow points to the
transducer. c) Screen capture of opened RF data
file showing a homogeneous ROI from mouse mammary
tissue. The dark region to the left of the ROI
is a lymph node.
4. In-Vivo Results Representative histology of
mouse mammary tissue (Fig. 5) shows the cellular
changes that occur during involution, as the
tissue changes from mostly epithelial cells to
predominantly fat. The KS goodness of fit test
reveals that the GG PDF provides a good fit at
all time points, while the Rayleigh PDF provides
a much poorer fit. The Rayleigh PDF provides the
best fit at day 2 of involution, coinciding with
the peak of apoptosis in the tissue (Fig. 6a).
The GG fit parameters show large changes between
days 1-3 (Fig. 6b). Over these days the GG fit
parameters approach values causing the GG PDF to
resemble a Rayleigh PDF.
a)
b)
c)
7. Acknowledgements We gratefully acknowledge the
assistance of Dr. John Hunt of OCI/PMH for his
assistance with the simulations. This work was
funded by Canadian Institutes of Health Research,
Natural Sciences and Engineering Research Council
of Canada and The Whitaker Foundation. The HFUS
scanner was purchased with funds from the Canada
Foundation for Innovation.
1mm
2mm