- PowerPoint PPT Presentation
1 / 39
Title:
Description:
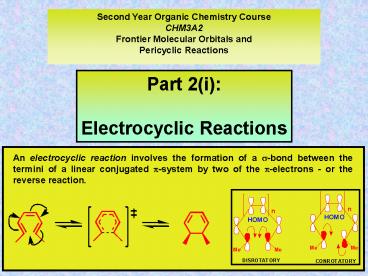
Second Year Organic Chemistry Course CHM3A2 Frontier Molecular Orbitals and Pericyclic Reactions Part 2(i): Electrocyclic Reactions An electrocyclic reaction involves ... – PowerPoint PPT presentation
Number of Views:36
Avg rating:3.0/5.0
Title:
1
(No Transcript)
2
Learning Objectives Part 2(i) Electrocyclic
Reactions
CHM3A2 Introduction to FMOs
After completing PART 2(i) of this course you
should have an understanding of, and be able to
demonstrate, the following terms, ideas and
methods. (i) An electrocyclic reaction involves
the formation of a ?-bond between the termini of
a linear conjugated ?-system by two of the
?-electrons - or the reverse reaction. (ii) Elect
rocyclic reactions are stereospecific. The
stereospecificity being afforded by the
disrotatory or conrotatory nature of the bond
making/breaking process (iii) 4?-electron
systems are conrotatory when thermally promoted,
(and disrotatory when photochemically promoted -
CHM3A2). (iv) 6?-electron systems are
disrotatory when thermally promoted (and
conrotatory when photochemically promoted -
CHM3A2). (v) The disrotatory or conrotatory
process involved in the bond making/breaking
process is controlled by the HOMO (thermal
reaction) or SOMO (photochemical reaction -
CHM3A2) of the linear conjugated ?-system which
either is the starting material or product.
3
6p-Electron Systems
RS
Meso
RR
Enantiomers
SS
4
4p-Electron Systems
RR
Enantiomers
SS
RS
Meso
5
HOMOs of Polyenes
A new s-bond is forming at the termini of each of
the polyene systems. Thus, it is clear that the
p-system of the polyene systems must be
interacting in some fashion. Analysis of the
polyenes has shown that by considering the HOMOs,
and rotating the termini of them to overlap them
in an in-phase fashion produces the correct
stereochemical outcome. The termini of the
orbitals can be rotated in two manners referred
to as Conrotatory, Disrotatory.
6
(No Transcript)
7
Disrotatory Motion Dark/Dark
In-phase
Meso
8
Disrotatory Motion Light/Light
In-phase
Meso
9
Conrotatory Motion Dark/Dark
In-phase
RS Enantiomer
10
Conrotatory Motion Light/Light
In-phase
SR Enantiomer
11
4n2 p Electron Electrocyclic Reactions
12
Dark/Dark Or Light/Light
RR and SS (enantiomers)
13
4n p Electron Electrocyclic Reactions
14
(No Transcript)
15
4n p Electron Electrocyclic Reactions
Conrotatory
16
Dark/Dark Or Light/Light
CONROTATORY
17
Enantiomer Formation
Two alternative and equivalent modes of
conrotatory in-phase overlap
18
(No Transcript)
19
Coping with Ring Opening Reactions
1. Draw out the p-HOMO of the product without
the substituents
?2 HOMO
20
2. Draw out the MO of the Starting material
?2 HOMO
21
3. Open the C-C bond two afford the HOMO of the
product
22
Product stereochemistry
4. Decide how the substituents move
23
Rules for Electrocyclic Reactions
__________________________________________________
_____Number of ?-Electrons Thermal Photochemic
al (CHM3A2) 4n
CONrotatory DISrotatory 4n 2
DISrotatory CONrotatory _________________
______________________________________
Photochemical reactions will be dealt with in the
third year course (CHM3A2), where the first
electronically excited stated state becomes the
HOMO.
24
Summary Sheet Part 2(i) Electrocyclic
Reactions
CHM3A2 Introduction to FMOs
An electrocyclic reaction involves the formation
of a ?-bond between the terminals of a linear
conjugated ?-system by two of the ?-electrons
or the reverse process. Electrocyclic reactions
are either 'allowed' or 'forbidden' and they
are stereospecific, occurring by either a
so-called conrotatory or disrotatory
motion. Electrocyclic reactions can be brought
about by heat (CHM2C3B), by ultraviolet
irradiation (CHM3A2), and sometimes by the use of
metal catalysts (CHM3A2). They are nearly
always stereospecific. In many cases, detection
of their stereospecificity depends on
distinguishing chemically similar stereoisomers -
a problem which has been overcome mainly by the
development of spectroscopic methods of structure
determination, especially NMR spectroscopy.
Thus, the recognition that stereospecific
electrocyclic reactions form a coherent group
extends only over the last quarter of a century.
Nowadays, the group includes some important
synthetic reactions as well as some of the most
clear cut examples of the successful predictive
power of orbital symmetry theory. In the case of
6p systems, the thermal ring closure of
1,3,5-hexatrienes to conjugated cyclohexadienes
is stereospecific - and disrotatory - as the
theory predicts. Ring closure of 1,3,
5-hexatrienes is a relative facile process
relative to butadiene ring closure which
generates a highly strained butadiene
derivatives. In the case of 4? systems, the
thermal ring opening of cyclobutenes to
butadienes is stereospecific - and conrotatory -
as the theory predicts. In most cases, the ring
opening goes to completion and there are very few
examples of the reverse process, the thermal
cyclisation of butadienes. Fused cyclobutenes,
however, are thermally rather stable, especially
those in which the second ring is five- or
six-membered.
25
Exercise 1 4n2 p Electrocylic Systems
The triene 1 undergoes a thermal electrocyclic
cyclisation. Using FMOs identify all the
products.
1
26
Answer 1 4n2 p Electrocylic Systems
The triene 1 undergoes a thermal electrocyclic
cyclisation. Using FMOs identify all the
products.
27
Exercise 2 4n2 p Electrocylic Systems
The two diastereoismeric trienes 1 and 2 undergo
thermal electrocyclic cyclisation reactions each
affording a pair of disubstituted conjugated
cyclic dienes. Identify all four products by
constructing the transition state geometries, and
state the stereochemical relationships that exist
between the pairs of stereoisomers formed from
each reaction and the stereochemical relationship
of the products between the pair of reactions
2
1
28
Answer 2 4n2 p Electrocylic Systems
The two diastereoismeric trienes 1 and 2 undergo
thermal electrocyclic cyclisation reactions each
affording a pair of disubstituted conjugated
cyclic dienes. Identify all four products by
constructing the transition state geometries, and
state the stereochemical relationships that exist
between the pairs of stereoisimers formed from
each reaction and the stereochemical relationship
of the products between the pair of reactions
Enantiomers
Enantiomers
Diasteroisomers
29
Exercise 3 4n p Electrocylic Systems
The cyclobutadiene derivative undergoes an
stereospecific electrocyclic ring opening
reaction to afford a single product. Utilise
FMOs to identify the product.
1
30
Answer 3 4n p Electrocylic Systems
The cyclobutadiene derivative undergoes an
stereospecific electrocyclic ring opening
reaction to afford a single product. Utilise
FMOs to identify the product.
31
Exercise 4 A Cascade Electrocylic System
Use FMOs to predict the stereochemical outcomes
in the reaction scheme below.
32
Answer 4 A Cascade Electrocylic System
Use FMOs to predict the stereochemical outcomes
in the reaction scheme below.
4n - CONROTATORY
(4n 2) - DISROTATORY
Dark/Dark
Dark/Dark
Light/Light
Light/Light
33
Exercise 5 Tandem Electrocyclic Reaction
Use FMOs to predict the stereochemical outcomes
in the reaction scheme right. In principle,
there are two possible products. Which will be
formed in highest yield. Justify your answer.
34
Answer 5 Tandem Electrocyclic Reaction
Use FMOs to predict the stereochemical outcomes
in the reaction scheme right. In principle,
there are two possible products. Which will be
formed in highest yield. Justify your answer.
The arrow pushing mechanism reveals that the
reaction involves the ring closure of two
1,3,5-hexatriene systems. Thus, need to consider
y3 HOMO of 1, 3, 5-hexatriene.
Light Light
Light Light
Thermodynamic Product. Least sterically hindered
Disrotatory of both triene systems
Light Light
Dark Dark
35
Exercise 6 Complex Electrocyclic Reaction
Cyclooctatetraene undergoes an electrocyclic ring
closure forming only the cis-isomer as depicted
right. Rationalise this result using FMOs.
36
Answer 6 Complex Electrocyclic Reaction
Cyclooctatetraene undergoes an electrocyclic ring
closure forming only the cis-isomer as depicted
right. Rationalise this result using FMOs.
4p Electron Process
CONROTATORY
6p Electron Process
DISROTATORY
Thus, the reaction must proceed by a 6 p electron
process, despite the 4 p electron process being
possible by FMO theory. Reasons for formation of
cis-isomer are possibly two-fold (i) cis-isomer
is the thermodynamically more stable product,
and/or (ii) the aromatic 6p electron aromatic
transition state is lower in energy than the 4p
electron anti-aromatic transition state.
37
Exercise 4n p Electrons Electrocyclic Reactions
Using FMOs rationalise why the two
diastereoisomers have such different
reactivities.
38
Answer 4n p Electrons Electrocyclic Reactions
Using FMOs rationalise why the two
diastereoisomers have such different
reactivities.
39
(No Transcript)