Defining Oxidation and Reduction - PowerPoint PPT Presentation
1 / 9
Title:
Defining Oxidation and Reduction
Description:
Defining Oxidation and Reduction Oxidation is the loss of electrons Reduction is the gain of electrons Ex. Consider the following reaction: – PowerPoint PPT presentation
Number of Views:427
Avg rating:3.0/5.0
Title: Defining Oxidation and Reduction
1
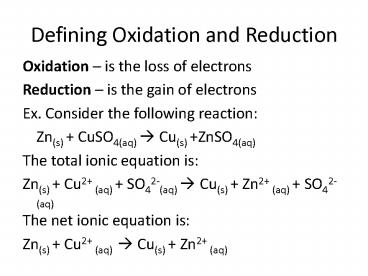
Defining Oxidation and Reduction
- Oxidation is the loss of electrons
- Reduction is the gain of electrons
- Ex. Consider the following reaction
- Zn(s) CuSO4(aq) ? Cu(s) ZnSO4(aq)
- The total ionic equation is
- Zn(s) Cu2 (aq) SO42-(aq) ? Cu(s) Zn2 (aq)
SO42-(aq) - The net ionic equation is
- Zn(s) Cu2 (aq) ? Cu(s) Zn2 (aq)
2
Defining Oxidation and Reduction
gains electrons
- Zn(s) Cu2 (aq) ? Cu(s) Zn2 (aq)
- In this reaction
- Zn atoms lose electrons ? oxidized ? zinc ions
- Cu ions gain electrons ? reduced ? Cu atoms
- In this case oxidation and reduction happen in
the same reaction so the reaction is called an
oxidation-reduction reaction or redox reaction.
loses electrons
3
Oxidizing and Reducing Agents
- Oxidizing Agents a reactant that oxidizes
another reactant, accepts electrons (causes the
other reactant to lose electrons) - Reducing Agents a reactant that reduces another
reactant, donates electrons (causes the other
reactant to gain electrons) - Zn(s) Cu2 (aq) ? Cu(s) Zn2 (aq)
- Zn is the reducing agent ? donates electrons ?
undergoes oxidation - Cu2 is the oxidizing agent ? accepts electrons ?
undergoes reduction
4
Practice Problems
- Pg. 653 2.
5
Half-Reactions
- To show the transfer of electrons in a chemical
reaction we often write the oxidation and
reduction separately. - Half-reaction a balanced chemical reaction that
shows the number of electrons involved in either
oxidation or reduction - Both half-reactions are required to represent a
redox reaction.
6
Representing Half-Reactions
- For the reaction of Zn with copper(II)sulfate
- The net ionic equation is
- Zn(s) Cu2(aq) ? Cu(s) Zn2(aq)
- The oxidation half-reaction is
- Zn(s) ? Zn2 (aq) 2e-
- The reduction half-reaction is
- Cu2(aq) 2e- ? Cu(s)
- In half-reactions, the charges and atoms are
balanced using coefficients
7
Half Reactions Continued
- Half-reactions always come in pairs
- 1 oxidation half-reaction
- 1 reduction half-reaction
- Why? We need both an electron donor and an
electron acceptor
8
Disproportionation Reactions
- Occur when a single element undergoes both
oxidation and reduction in the same reaction - Some of the reactant molecules are oxidized, some
are reduced - Ex. Disproportionation of a copper(I) solution
- 2Cu(aq) ? Cu(s) Cu2(aq)
- Oxidation half-reaction Cu(aq) ? Cu2(aq) 1e-
- Reduction half-reaction Cu (aq) 1e- ? Cu(s)
9
Practice Problems/ Section Review
- Practice Problems
- Complete Pg. 656 8, 9