Sedimentary Materials - PowerPoint PPT Presentation
1 / 25
Title:
Sedimentary Materials
Description:
Once we weather the source material, the material is ... (a.k.a. clastic) form by compaction and lithification of clastic sediments or lithic fragments ... – PowerPoint PPT presentation
Number of Views:43
Avg rating:3.0/5.0
Title: Sedimentary Materials
1
Sedimentary Materials
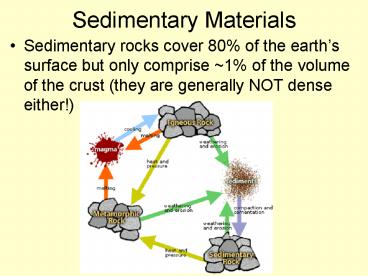
- Sedimentary rocks cover 80 of the earths
surface but only comprise 1 of the volume of
the crust (they are generally NOT dense either!)
2
- Once we weather the source material, the material
is transported, deposited, compacted, and
lithified, and maybe changed by reaction with
groundwater (called diagenesis)
3
Transport
- All weathered products can be transported
- Dissolved ions are transported until they get to
a final destination (such as the ocean) and/ or
are precipitated - Physically weathered minerals/ rock fragments ?
How are they transported? - Water, wind, glaciers, gravity
- What processes are more selective to the size of
the particle
4
Types of sedimentary rocks
- Detrital (a.k.a. clastic) ? form by compaction
and lithification of clastic sediments or lithic
fragments - Clasts are little grains or fragments of rocks
(i.e. can be made of 1 or more minerals) - Classification based on size
- Chemical ? form by precipitation of minerals from
water, or by alteration of pre-existing material - Classification based on chemical composition
- Biogenic ? formed of previously living organic
debris - HOWEVER ? Many sedimentary rocks are combinations
of 2-3 of these types WHY?
5
Weathering
- Looking at the rock cycle, key to forming
sedimentary rocks is weathering (or erosion) of
pre-existing rocks (or organisms) - Types of weathering
- Physical (a.k.a. mechanical)
- Chemical
6
Physical Weathering
- Joints and sheeting development in rocks
- Frost wedging, salt wedging, biologic wedging
- Thermal stress
- Abrasion through water, wind, glaciers,
gravity, waves
7
Exfoliation or unloading
- Some rocks expand to to pressure release, uplift,
heating/ cooling, etc. and break off in sheets
8
Chemical Weathering
- How do we dissolve stuff?
- Ions dissolve into water based on properties of
that ion and how easily the mineral releases it
into the water - What properties do you think make the ions in a
mineral dissolve more easily?
Fe2
SiO2
olivine
Mg2
SiO2
9
Chemical Weathering Vocabulary
- Hydrolysate dissolved material
- Resistate solid material left behind (didt
dissolve) - More easily dissolved elements include alkali and
alkaline earths (Na, Ca2, K) - Residual product of hydrolysis reactions left
behind (it can be physically weathered too)
10
Mineral Dissolution
- Write a reaction
- Mg0.5Fe0.5SiO4 H2O ? 0.5 Mg2 0.5 Fe2
SiO44- - Describe that reaction as an equilibrium
expression which defines how much of the mineral
can dissolve in a particular fluid - What aspects of fluid composition do you think
might affect how much of a mineral can dissolve? - Keqproducts / reactants
- KeqMg2Fe2SiO44- / olivineH2O
11
Aqueous Species
- Dissolved ions can then be transported and
eventually precipitate - Minerals which precipitate from solution are
rarely the same minerals the ions dissolved out
of - Why would they be transported before
precipitating?
K
SiO2
smectite
feldspar
Na
SiO2
12
Chemical Weathering II - hydrolysis
- Some minerals weather directly to other
minerals - Mineral dissolves and immediately reprecipitates
a new mineral at the surface of the original - Feldspars ? Clays
- Fe-bearing silicates to iron oxyhydroxides
olivine
olivine
FeOOHs
13
Acid/base reactions
- Many minerals are affected by the pH of the
solution they are in - some form H or OH- when they dissolve
- Some dissolve much faster/ better in low or high
pH solutions - Calcite weathering
- CaCO3 H H2O ? H2CO3(g) CaOH
- Acid/ base chemistry important in mineral
dissolution and precipitation!!
14
Oxidation
- Recall that elements exist as different ions in a
particular oxidation state - Changing that oxidation state can have a big
effect on how well that element will dissolve and
what minerals will form after it dissolves - Oxidation (where a reduced ion loses an electron
to an oxidant) is important in the weathering of
many minerals at the surface of the earth where
O2 is the oxidant - Fe(II)2SiO4 ½ O2 H2O ? 2 Fe(III)OOH SiO2
15
Chemical Weathering
- Recap How do minerals dissolve?
- Dissolution reactions
- Ions dissolve in water, do not change
- Acid-base reactions
- Ions dissolve in water through interaction with
H or OH- - Redox reactions
- Ions dissolve/ precipitate affected by
interaction of ions in mineral or in water with O2
16
Chemical Weathering and Stability
- All minerals are described by a stability
- Thermodynamics defines this through an energy ?
all energies are relative - Energy changes depending on the conditions ? i.e.
some minerals are more stable than others at high
P and T change the P and T conditions and
different minerals are more stable - In weathering environments,
- minerals that are weathering
- are not stable, minerals
- precipitating ARE stable
17
- Examples of graphical representations of mineral
stability derived from thermodynamic calculations
18
Resistance to weathering
- Goldrich series ? empirical observation
concerning what minerals weather before others
Remind you of anything??
19
What happens when granite is weathered??
- First, unweathered granite contains these
minerals - Na Plagioclase feldspar
- K feldspar
- Quartz
- Lesser amounts of biotite, amphibole, or
muscovite - What happens when granite is weathered?
- The feldspars will undergo hydrolysis to form
kaolinite (clay) and Na and K ions - The Na and K ions will be removed through
leaching - The biotite and/or amphibole will undergo
hydrolysis to form clay, and oxidation to form
iron oxides.
20
Granite weathering, continued
- The quartz (and muscovite, if present) will
remain as residual minerals because they are very
resistant to weathering. - Weathered rock is called saprolite.
- What happens after this?
- Quartz grains may be eroded, becoming sediment.
The quartz in granite is sand- sized it becomes
quartz sand. The quartz sand will ultimately be
transported to the sea (bed load), where it
accumulates to form beaches. - Clays will ultimately be eroded and washed out to
sea. Clay is fine-grained and remains suspended
in the water column (suspended load) it may be
deposited in quiet water. - Dissolved ions will be transported by rivers to
the sea (dissolved load), and will become part of
the salts in the sea.
21
Sedimentary Minerals
- We will focus on some minerals which form from
precipitation of dissolved ions ? other minerals
in sedimentary rocks are derived from the source
rocks! - Clay, carbonate, and sulfate groups are key in
sedimentary rocks can be the rock or cement
fragments together! - SiO44-, CO32-, SO42- anionic groups, respectively
- Also consider halides (anion is Cl- or F-) and
mineralization of silica
22
Clays
- Sheet Silicates aka Phyllosilicates
Si2O52- Sheets of tetrahedra
Phyllosilicates micas
talc clay minerals serpentine
23
- Sheet Silicates aka Phyllosilicates
- Si2O52- Sheets of tetrahedra
Phyllosilicates - micas talc clay minerals serpentine
- Clays ? talc ? pyrophyllite ? micas
- Display increasing order and lower variability of
chemistry as T of formation increases
24
Clays
- Term clay ALSO refers to a size (lt 1mm lt10-6 m)
- Sheet silicates, hydrous some contain up to 20
H2O ? together with a layered structure and weak
bonding between layers make them SLIPPERY WHEN
WET - Very complex (even argued) chemistry reflective
of specific solution compositions
25
Major Clay Minerals
- Kaolinite Al2Si2O5(OH)4
- Illite K1-1.5Al4(Si,Al)8O20(OH)4
- Smectites
- Montmorillonite (Ca, Na)0.2-0.4(Al,Mg,Fe)2(Si,Al
)4O10(OH)2nH2O - Vermicullite - (Ca, Mg)0.3-0.4(Al,Mg,Fe)3(Si,Al)4O
10(OH)2nH2O - Swelling clays can take up extra water in their
interlayers and are the major components of
bentonite (NOT a mineral, but a mix of different
clay minerals)