Cpt 5' STEREOCHEMISTRY - PowerPoint PPT Presentation
1 / 25
Title:
Cpt 5' STEREOCHEMISTRY
Description:
Chirality center = asymetric center: a C that is bound to 4 different atoms or groups of atoms ... Chirality of Substituted Cyclohexanes ... – PowerPoint PPT presentation
Number of Views:111
Avg rating:3.0/5.0
Title: Cpt 5' STEREOCHEMISTRY
1
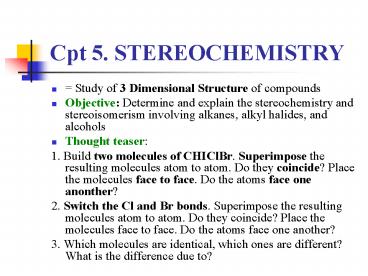
Cpt 5. STEREOCHEMISTRY
- Study of 3 Dimensional Structure of compounds
- Objective Determine and explain the
stereochemistry and stereoisomerism involving
alkanes, alkyl halides, and alcohols - Thought teaser
- 1. Build two molecules of CHIClBr. Superimpose
the resulting molecules atom to atom. Do they
coincide? Place the molecules face to face. Do
the atoms face one anonther? - 2. Switch the Cl and Br bonds. Superimpose the
resulting molecules atom to atom. Do they
coincide? Place the molecules face to face. Do
the atoms face one another? - 3. Which molecules are identical, which ones are
different? What is the difference due to?
2
5.1. Stereoisomerism
- Stereoisomers Isomers that differ only by their
configuration (the way their bonds are arranged
in space). - Ex
- HCI(Br)(Cl)
- Cis-Trans isomers
3
5.2. Enantiomerism
- Enantiomers two stereoisomers that are mirror
images of one another - Ex HCI(Br)(Cl)
- All enantiomers are Chiral (Handed) not
superimposable with one another - ex 2
- CH3CHBrCH2CH3
- CH3CBr2CH2CH3
- Chirality center asymetric center a C that is
bound to 4 different atoms or groups of atoms - Contributes to the chirality of the molecule
4
5.3. Optical Activity (Specific Rotation)
- Resource Ctr Slide 15-17
- Components of normal light oriented in all
directions - Components of Plane-Polarized lightdirected only
in one plane (direction) - Polarizer lens that lets through light that is
oriented in only one direction and blocks light
oriented in all other directions - Chiral molecules change direction of polarized
light
5
Optical activity (Continued)
- Polarimeter instrument used to measure optical
activity - Optical activity ability to change direction of
polarized light - Optical Rotation angle by which a chiral
compound turns the direction of polarized light. - Dextrorotatory compounds that rotate polarized
light to the right - Levorotatorycompounds that rotate polarized
light to the left
6
Optical activity (Continued 2)
- Specific Rotation of degrees by which
polarized light is rotated by a sample of
substance at 1 g/ml concentration inside a 1 dm
long optical rotation measuring tube - aD a / (l x C)
- a specific rotation
- a observed rotation
- l length of measuring tube(dm)
- d density for pure liquids or concentration for
solutions (g/ml) - ex 5-9, pg 183
7
Optical activity (Continued 3)
- Enantiomers have opposite optical activities.
- Ex 2-butanol
- 13.5 o for the D enantiomer
- - 13.5 o L
- Most chemical and physical properties of
enantiomers are identical. Ex mp, bp, Rf,
8
5.4. Absolute Configuration R S
- Definition Systematic way to assign the
stereochemistry of molecules. - Thought teaser Which one of the 2-butanol
enantiomers is D? - Cahn-Ingold-Prelog Configuration Determination
Procedure - Step 1 set chiral center with lightest atom (H)
or group of atoms oriented away from you - Step 2 number the remaining atoms (or groups of
atoms) from the heaviest to the lightest - If numbering clockwise configuration R
- If numbering anticlockwise configuration S
9
R S Configuration(Illustrations)
10
R S Configuration(Illustrations 2)
11
R S Configuration(Exercises)
12
R and S Configuration (Continued)
- Special cases
- Double and triple bonds
- atoms involved in double bonds are duplicated
- atoms involved in triple bonds are triplicated
13
Absolute configuration (Special Cases
Illustration)
14
Fisher projections
- Fisher projections simple representation of
chiral compounds - Horizontal lines used for bonds directed
toward us - Vertical lines used for bonds directed away
from - Molecule chain must be set in eclipsing
conformation. - Determine the stereochemistry of Fisher
projections one atom at a time.
15
Fisher projections (Examples)
16
5.5. Diastereomers Meso Compounds
- Diastereomers chiral isomers which are not
mirror images of one another. - ex 2R,3R,4R,5S-2,3,4,5-hexanetetraol, and
2R,3R,4R,5R-2,3,4,5-hexanetetraol. - Meso compounds nonchiral molecules that have
chiral centers. Reason for nonchirality symmetry
of the molecule. Apparent meso isomers are
identical molecules. - ex 2R,3R,4S,5S-2,3,4,5-hexanetetraol.
17
Diastereomers Meso Compounds (Continued)
18
Diastereomers Meso Compounds (Exercises)
19
5.6. Chirality of Substituted Cyclohexanes
- Thought teaser Build the models of
1-ethyl-4-methylcyclohexane and
1-ethyl-3-methylcyclohexane. Which molecule is
symmetrical? - Principles the following molecules are achiral
- Symmetrical molecules
- Molecules that rapidly convert to their
enantiomers - Molecules in all other cases are chiral
- Ex
- 1,2-disubstituted cyclohexanes
- 1,3-disubstituted cyclohexanes
20
5.7. Optical Purity (OP) Enantiomeric Excess
(EE).
- OP and EE measure the purity of non racemic
mixtures of enantiomers. - OP ratio of rotation of mixture to rotation of
pure enantiomer - OP a(observed)/a (pure) x 100
- EE Wt(d) - Wt(l)/Wt(d) Wt(l) x 100
- Ex(35-12, pg 187)
21
Optical Purity (Example)
- Problem When R-2-bromobutane is heated with
water, a mixture of 2x as much S-2-butanol as
R-2-butanol is formed. Calculate the EE and the
specific rotation of expected for the product. - Info provided Proportion of S-2-butanol (a
13.5o) 2x as much as R-2-butanol. - Info requested EE and the specific rotation of
expected for the product. - Solution EE (2-1)/(21) X 100 33.33
- a 13.5o x 33.33/100 4.5o
22
Optical Purity (Example 2)
- Problem The specific rotation of pure
(-)-2-butanol is -13.5o. What of a mixture of
the two enantiomeric forms is the (-)-form if the
specific rotation of this mixture is 7.0o? - Info provided
- a of (-)-2-butanol -13.5o
- a of a mixture of (-) and ()-2-butanol
7.0o - Info requested of ()-2-butanol
23
Optical Purity (Solution)
- OP (a(observed) / a(pure)) x 100 of the
majority enantiomer - of the minority enantiomer 100 - of the
majority enantiomer
24
Optical Purity (Exqmple 3)
- Problem (-)-Lactic acid has a specific rotation
of -3.8o. What is the specific rotation of a
solution containing 7.5 g of (-)-lactic acid and
2.5 g of ()-lactic acid? - Info provided
- a of (-)-lactic acid -3.8o
- Wt of (-) lactic acid 7.5 g
- Wt of () lactic acid 2.5 g
- Info requested a of the mixture of (-) and ()
lactic acids.
25
Optical Purity (Solution3)
- a(observed) OP x a(pure) / 100
- OP EE 100 x (d l) / (dl)