Metabolism breaking molecules down and building up new ones - PowerPoint PPT Presentation
1 / 29
Title:
Metabolism breaking molecules down and building up new ones
Description:
All the C from the glucose is now oxidized to CO2. ... can oxidize S to SO42- in aerobic conditions or reduce S to H2S in presence of ... – PowerPoint PPT presentation
Number of Views:45
Avg rating:3.0/5.0
Title: Metabolism breaking molecules down and building up new ones
1
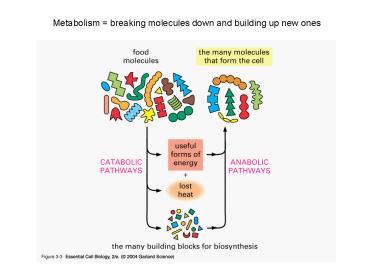
Metabolism breaking molecules down and building
up new ones
2
Important processes in metabolism Discuss
processes in order in which they (might have)
evolved
- Anaerobic breakdown of organic molecules
fermentation. Fits with
primordial soup argument (first organisms
heterotrophic). - Respiration electron transport chains (still
heterotrophs but much more efficient). - Chemosynthesis (autotrophs can carry out carbon
fixation. No longer limited by the soup). - Photosynthesis (autotrophs huge amounts of
energy for free! Major increase in biomass).
3
Glycolysis breakdown of sugar
Essentials worth remembering 1 glucose (6C) ? 2
pyruvate (3C) Generates 2 ATP and 2 NADH
4
Essentials In anaerobic bacteria pyruvate is
broken down to waste products (e.g.
lactate). NAD is regenerated (a cycle)
also occurs in muscles
Other examples of fermentation processes Pyruvate
? CO2 ethanol Pyruvate ? CO2 acetic
acid These occur in yeast
CO2
Glucose is only partly oxidized by these
reactions. Relatively inefficient.
5
In aerobic organisms, pyruvate feeds into the
Citric Acid Cycle (Krebs cycle)
Acetyl CoA
Essentials This produces NADH and FADH2. These
are electron donors (reducing agents) for the
electron transport chain. All the C from the
glucose is now oxidized to CO2. Many other
biosynthetic pathways branch off from glycolysis
and citric acid cycle.
6
Important processes in metabolism Discuss
processes in order in which they (might have)
evolved
- Anaerobic breakdown of organic molecules
fermentation. Fits with
primordial soup argument (first organisms
heterotrophic). Relatively simple.
- Respiration electron transport chains (still
heterotrophs but much more efficient). Really
clever, but complicated. - Chemosynthesis (autotrophs can carry out carbon
fixation. No longer limited by the soup). - Photosynthesis (autotrophs huge amounts of
energy for free! Major increase in biomass).
7
Oxidation-Reduction again -
FAD
Flavin adenine dinucleotide
FADH2
FADH2 ? FAD 2H 2e-
NADH ? NAD H 2e-
Now we are going to make use of those electron
donors we just made two slides back. Hang onto
your hats!
8
2e-
H2O
NADH
ubiquinone
cytochrome c
2H ½ O2
NAD
NADH dehydrogenase complex
cytochrome b-c1 complex
cytochrome oxidase complex
Essentials Aerobic respiration (in aerobic
bacteria or in mitochondria in eukaryotes) High
energy electron donor eventually donates
electrons to O2 Electron goes downhill in
?G Proton gradient is generated.
heme group in cytochrome c
9
ATP synthetase complex
proton channel
Electron transport chain ATP synthesis
oxidative phosphorylation
chemiosmotic process For each molecule of glucose
about 30 ATPs generated by ox. phos. but only 2
from glycolysis. Much more energy from the same
food!
ADP Pi
protons moving downhill provide energy for uphill
synthesis of ATP
ATP
10
Other respiratory chains In each case organic
molecules are oxidized. The terminal electron
acceptor is reduced. The energy released is used
to generate a proton gradient that is used for
ATP synthesis. In aerobic respiration O2 is the
electron acceptor. In anaerobic respiration
another molecule is the electron acceptor.
A Archaea B Bacteria can also be
chemoautotrophic
11
Evolution of respiratory chains
Early organisms probably used fermentation only
(anaerobic). Fermentation usually leads to
excretion of acids (lactic, formic,
acetic....). Proton pump would be favoured to
keep the acid out.
ATP synthase works both ways. May have originated
as an ATP driven proton pump.
Electron transport chain enabled H to be pumped
without using ATP.
If electron transport chain pumps became more
efficient than necessary, the proton gradient
could be used to drive ATP synthase to make ATP.
H
ADP Pi ? ATP
12
Important processes in metabolism Discuss
processes in order in which they (might have)
evolved
- Anaerobic breakdown of organic molecules
fermentation. Fits with
primordial soup argument (first organisms
heterotrophic). ? Relatively simple.
Maybe these kind of reactions were catalyzed by
ribozymes in the RNA world. NADH, FADH2, CoA all
involve nucleotides (clue?). - Respiration electron transport chains (still
heterotrophs but much more efficient). Really
clever, but complicated. Each complex in the
respiratory chain involves many proteins. No RNAs
known to do this. ? probably this comes after RNA
world but before LUCA ? Now we
can efficiently generate energy from food, but we
are running out of food... - Chemosynthesis (autotrophs can carry out carbon
fixation. No longer limited by the soup). - Photosynthesis (autotrophs huge amounts of
energy for free! Major increase in biomass).
13
Chemoautotrophy (Chemolithotrophy) An inorganic
reducing agent feeds into an electron transport
chain. Generates a proton gradient (more ATP
synthesis) and an organic reducing agent (like
NAD(P)H), which reduces CO2 to organic molecules.
Several different carbon fixation cycles are
known opposite of citric acid cycle.
14
- Essentials
- Many possible energy sources from redox
reactions. - Can go both ways - 2 examples
- can oxidize S to SO42- in aerobic conditions or
reduce S to H2S in presence of H2 gas but absence
of O2 ---- both have ?G lt 0 in the right
conditions. - methylotrophy (aerobic) v. methanogenesis
(anaerobic)
Sometimes the same organism goes both ways e.g.
Sulfolobus can be an anaerobic heterotroph with
sulphur reduction, or an autotrophic aerobic
sulphur oxidizer
clever cloggs!
Redox reactions in previous table have ?G lt 0.
They look simple, but remember they dont just
happen in one step as an inorganic reaction.
These reactions are coupled to electron
transport chains and proton gradients....
15
Important processes in metabolism Discuss
processes in order in which they (might have)
evolved
- Anaerobic breakdown of organic molecules
fermentation. Fits with
primordial soup argument (first organisms
heterotrophic). ? Relatively simple.
Maybe occurred in the RNA world. - Respiration electron transport chains (still
heterotrophs but much more efficient). Really
clever, but complicated. Each complex in the
respiratory chain involves many proteins. No RNAs
known to do this. ? probably this comes after RNA
world but before LUCA - Chemosynthesis (autotrophs can carry out carbon
fixation. No longer limited by the soup).
? Many possible
sources of chemical energy.
? Some of these
types of metabolism are found in both archaea and
bacteria, i.e. before LUCA. - Photosynthesis (autotrophs huge amounts of
energy for free! Major increase in biomass).
? Only in bacteria,
i.e. after LUCA
? requires
light-harvesting protein complexes (photosystems)
16
Complementary processes of photosynthesis and
respiration
Carbon fixation into sugars reduction of CO2
Oxidation of sugars into CO2
(In anaerobic organisms sugars are oxidized
incompletely via fermentation. O2 not required.)
(Some forms of photosynthesis do not produce
oxygen)
17
Two types of chlorophyll absorb visible light at
slightly different wavelengths. Chlorophyll
contained in the photosystem I and II protein
complexes
high energy electron enters the transport chain
low energy electron replaces it
light excites an electron
delocalized electrons in ring structure
18
Photosynthesis a light-driven electron transport
chain
Thylakoid membrane of chloroplasts (or outer
membrane of photosynthetic bacteria)
light
light
H2O
2H ½ O2
H
2e-
NADPH
plastoquinone
plastocyanin
ferredoxin
H
NADP
Photosystem II
cytochrome b6-f complex
Photosystem I
Ferredoxin-NADP reductase
Generates proton gradient that can be used by ATP
synthase
NADPH is a reducing agent that can reduce CO2 to
organic molecules
19
The dark reactions of photosynthesis. Carbon
fixation cycle (Calvin cycle). CO2 is reduced to
sugars. Requires energy and reducing power.
20
Types of photosynthesis 5 groups of bacteria
perform photosynthesis. In oxygenic
photosynthesis H2O is the electron donor and O2
is produced. In anoxygenic photosynthesis H2S is
the electron donor and O2 is not produced.
21
Evolution of photosynthesis (see Olsen and
Blankenship, 2004)
PS I Chlorobium and Heliobacteria
divergence in separate lineages
endosymbiosis chloroplasts
fusion
PS I II Cyanobacteria
ancestral PS
PS II Chloroflexus and Purple bacteria
PSs contain different types of chlorophyll. Genes
for pigment synthesis may not follow same tree as
genes for the components of the PSs. Evidence for
horizontal transfer. Archaea do not have these
photosystems. They evolved after the LUCA.
However Halobacteria (which are salt-loving
extremophile archaea) have an independent light
harvesting protein called bacteriorhodpsin in
their purple membrane. Contains retinal
chromophore. Different to chlorophyll.
22
Plausible summary of Everything
Bacteria
Archaea
Eukaryotes
chloroplasts
Oxygenic Photosynthesis
Methanogenesis/ Bacteriorhodopsin only in Archaea
mitochondria
Anoxygenic Photosynthesis
origin of eukaryotic nucleus ?
Genes for sulphate reduction, nitrate reduction,
sulphur oxidation, oxygen respiration all present
in A and B
LUCA
Chemosynthesis Electron transport chains
Simple heterotrophic metabolism / fermentation
Origin of life
23
Alternative viewpoint 1 Early evolution of
photosynthesis Mauzerall argues that only
photosynthesis could supply sufficient energy for
life. Light absorbing pigments must have existed
very early. These would have initiated redox
reactions. But these would be independent of
todays membrane bound electron transport
chains. ?? But some proteins in the respiratory
and photosynthetic chains are related. Suggests
that (current form of) photosythesis was later.
Alternative viewpoint 2 Chemoautotrophic
origin Wächtershäuser argues that an autotrophic
metabolism based on pyrite was first. FeS H2S ?
FeS2 H2 ?? This may be a plausible energy
source but (current forms of) autotrophs use
complex electron transport pathways. If this
existed, evidence of it is lost. ?? The first
organisms must have been made of something!
Presumably organic molecules .... This brings us
back to the primordial soup....
Alternative viewpoint 3 Clay mineral
origin Cairns-Smith argues that organic molecules
were not important originally. Clay minerals
stored information. Genetic takeover occurred
(e.g. to RNA).
24
Extremophiles
What counts as extreme? Depends on our
viewpoint. What limits organisms? Challenges in
different environments. How to overcome
them? What can they tell us about possibility of
life elsewhere?
Congress pool. Yellowstone. pH3
80oC Sulfolobus acidocaldarius
Pictures from Rothschild Mancinelli (2001) See
also Lunine Chap 10 Chapters by Rothschild and
Stetter in OI book.
25
Temperature
gt80 Hyperthermophiles
60-80 Thermophiles
15-60 Mesophiles
lt15 Psychrophiles
Eukaryotes more limited at high temp than
bacteria and archaea Low temp organisms from all
domains
Growth rate measurements distinguish tolerant
organisms from true philes
26
Challenges of high T stability of molecular
structures, membranes, and molecules themselves
Examples of
molecular adaptation to high T
In proteins ?Gunfolding found to be large in
thermozymes Tunfolding is higher More hydrogen
bonds with water. More salt bridges between and
charged residues. More disulphide bonds between
cysteines. Folded structures more rigid, fewer
cavities.
But overall genomic GC content does not correlate
with T. DNA must be stable anyway...
27
Psychrophiles challenges of low temps Membrane
becomes too rigid need to change lipid
structure Slows down reaction rates Liquid water
usually required for reactions Ice crystals
expand relative to water can tear cells apart.
Antifreeze proteins found in fish that live at lt
0 Small helical proteins can bind to the surface
of small ice crystals and prevent them growing.
Sea-ice diatoms (unicellular photosynthetic
eukaryotes)
28
Salinity Halophiles Salt conc in ocean is 3.5,
but this is too high for us. Some organisms are
adapted to concs up to 35 in salt lakes
Water will diffuse out of the cell by osmosis.
Causes dessication. Many halophiles use
Compatible solutes - small organic molecules
that do not interfere with metabolism when
accumulated to high conc. Extreme halophiles use
salt-in-cytoplasm K are selectively allowed
into cell to balance the osmotic pressure.
Enzymes have to adjust to working in this
situation.
Halobacteria in a salt lake (Archaea with
photosynthetic purple membrane)
29
(No Transcript)