Fluorescence, Phosphorescence, - PowerPoint PPT Presentation
1 / 21
Title:
Fluorescence, Phosphorescence,
Description:
Excitation of e- by absorbance of hn. - Re-emission of hn as e- goes ... oxidize compound ' paramagnetic property increase intersystem crossing (spin flipping) ... – PowerPoint PPT presentation
Number of Views:463
Avg rating:3.0/5.0
Title: Fluorescence, Phosphorescence,
1
Fluorescence, Phosphorescence,
Chemiluminescence
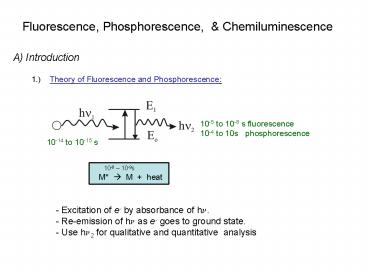
A) Introduction 1.) Theory of Fluorescence and
Phosphorescence
10-5 to 10-8 s fluorescence 10-4 to 10s
phosphorescence
10-14 to 10-15 s
- - Excitation of e- by absorbance of hn.
- - Re-emission of hn as e- goes to ground state.
- Use hn2 for qualitative and quantitative
analysis
2
Fluorescence, Phosphorescence,
Chemiluminescence
A) Introduction 1.) Theory of Fluorescence and
Phosphorescence
For UV/Vis need to observe Po and P difference,
which limits detection
For fluorescence, only observe amount of PL
3
2.) Fluorescence ground state to single state
and back. Phosphorescence - ground state to
triplet state and back.
10-5 to 10-8 s
10-4 to 10 s
Spins unpaired net magnetic field
Spins paired No net magnetic field
4
3) Jablonski Energy Diagram
S2, S1 Singlet States
T1 Triplet State
Numerous vibrational energy levels for each
electronic state
Resonance Radiation - reemission at same
l usually reemission at higher l (lower energy)
Forbidden transition no direct excitation of
triplet state because change in multiplicity
selection rules.
5
4.) Deactivation Processes a) vibrational
relaxation solvent collisions - vibrational
relaxation is efficient and goes to lowest
vibrational level of electronic state
within 10-12s or less. - significantly shorter
life-time then electronically excited state -
fluorescence occurs from lowest vibrational level
of electronic excited state, but can go to
higher vibrational state of ground level. -
dissociation excitation to vibrational state
with enough energy to break a bond -
predissociation relaxation to vibrational state
with enough energy to break a bond
6
4.) Deactivation Processes b) internal
conversion not well understood - crossing
of e- to lower electronic state. - efficient
since many compounds dont fluoresce -
especially probable if vibrational levels of two
electronic states overlap, can lead to
predissociation or dissociation.
7
4.) Deactivation Processes c) external
conversion deactivation via collision with
solvent (collisional quenching) - decrease
collision ? increase fluorescence or
phosphorescence decrease temperature and/or
increase viscosity decrease concentration
of quenching (Q) agent.
Quenching of Ru(II) Luminescence by O2
8
4.) Deactivation Processes d) intersystem
crossing spin of electron is reversed -
change in multiplicity in molecule occurs
(singlet to triplet) - enhanced if
vibrational levels overlap - more common if
molecule contains heavy atoms (I, Br) - more
common in presence of paramagnetic species
(O2)
9
5.) Quantum Yield (f) ratio of the number of
molecules that luminesce to the total
number of excited molecules. -
determined by the relative rate constants (kx) of
deactivation processes f kf
kf ki kec kic
kpd kd f fluorescence I
intersystem crossing ec external conversion
ic internal conversion pd
predissociation d dissociation
Increase quantum yield by decreasing factors that
promote other processes
Fluorescence probes measuring quantity of protein
in a cell
10
6.) Types of Transitions - seldom occurs
from absorbance less than 250 nm
200 nm gt 600 kJ/mol, breaks many bonds -
fluorescence not seen with s ? s - typically
p ? p or p ? n
11
7.) Fluorescence Structure - usually
aromatic compounds low energy of p ?p
transition quantum yield increases with
number of rings and degree of
condensation. fluorescence especially
favored for rigid structures lt fluorescence
increase for chelating agent bound to
metal.
Examples of fluorescent compounds
quinoline indole fluorene 8-hydroxyquinoline
12
8.) Temperature, Solvent pH Effects -
decrease temperature ? increase
fluorescence - increase viscosity ? increase
fluorescence - fluorescence is pH dependent
for compounds with acidic/basic
substituents. more resonance forms
stabilize excited state.
Fluorescence pH Titration
resonance forms of aniline
13
9.) Effect of Dissolved O2 - increase O2
? decrease fluorescence oxidize
compound paramagnetic property increase
intersystem crossing (spin flipping)
Change in fluorescence as a function of cellular
oxygen
Am J Physiol Cell Physiol 291 C781C787, 2006.
14
B) Effect of Concentration on Fluorescence or
Phosphorescence power of fluorescence
emission (F) KPo(1 10 ebc)
K f (quantum yield) Po
power of beam ebc Beers law
F depends on absorbance of light and incident
intensity (Po) At low concentrations F
2.3KebcPo deviations at higher concentrations
can be attributed to absorbance becoming a
significant factor and by self-quenching or
self-absorption.
Fluorescence of crude oil
15
C) Fluorescence Spectra Excitation Spectra (a)
measure fluorescence or phosphorescence at a
fixed wavelength while varying the excitation
wavelength. Emission Spectra (b)
measure fluorescence or phosphorescence over a
range of wavelengths using a fixed
excitation wavelength.
Phosphorescence bands are usually found at longer
(gtl) then fluorescence because excited triple
state is lower energy then excited singlet state.
16
D) Instrumentation - basic design components
similar to UV/Vis spectrofluorometers
observe both excitation emission
spectra. - extra features for
phosphorescence sample cell in cooled Dewar
flask with liquid nitrogen delay between
excitation and emission
17
Fluorometers - simple, rugged, low cost,
compact - source beam split into reference and
sample beam - reference beam attenuated
fluorescence intensity
A-1 filter fluorometer
18
Spectrofluorometer - both excitation and
emmision spectra - two grating monochromators
- quantitative analysis
Perkin-Elmer 204
19
E) Application of Fluorescence - detect
inorganic species by chelating ion
8-Hydroxyquinoline flavanol
alizarin garnet R benzoin
20
F) Chemiluminescence - chemical reaction yields
an electronically excited species that emits
light as it returns to ground state. -
relatively new, few examples A B ? C ? C
hn Examples
- Chemical systems
- - Luminol (used to detect blood)
- - phenyl oxalate ester (glow sticks)
21
2) Biochemical systems - Luciferase (Firefly
enzyme)
Glowing Plants Luciferase gene cloned into
plants
Luciferin (firefly)