Polysaccharides - PowerPoint PPT Presentation
1 / 32
Title:
Polysaccharides
Description:
Chitin polymer of glucosamine (an amino sugar), found in the exoskeleton of bugs. ... Exoskeleton of insects. Polysaccharides Digestion. Glucose Polymers ... – PowerPoint PPT presentation
Number of Views:622
Avg rating:3.0/5.0
Title: Polysaccharides
1
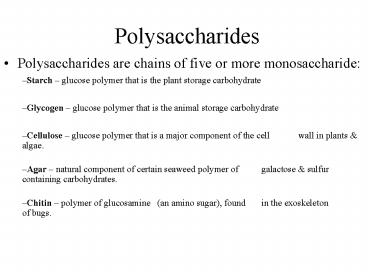
Polysaccharides
- Polysaccharides are chains of five or more
monosaccharide
- Starch glucose polymer that is the plant
storage carbohydrate
- Glycogen glucose polymer that is the animal
storage carbohydrate
- Cellulose glucose polymer that is a major
component of the cell wall in plants
algae.
- Agar natural component of certain seaweed
polymer of galactose sulfur containing
carbohydrates.
- Chitin polymer of glucosamine (an amino
sugar), found in the exoskeleton of bugs.
2
Starch
- Starch is used for energy storage in plants
- Two types amylose and amylopectin. On complete
hydrolysis each type gives only D-glucose
- Amylose is composed of continuous, unbranched
chains of up to 4000 D-glucose units joined by
a-1,4-glycoside bonds
- Amylopectin is a highly branched polymer of
D- glucose. Chains consist of 24-30 units of
D- glucose joined by a-1,4-glycoside bonds and
branches created by a-1,6-glycoside bonds
3
Amylose
- Soluble starch, polymer of D-glucose.
- Starch-iodide complex, deep blue.
4
Amylopectin
- Branched, insoluble fraction of starch.
A-1,4-glycosidic linkage
5
Glycogen
The total amount of glycogen in the body of a
well-nourished adult is about 350 g (about 3/4 of
a pound) divided almost equally between liver and
muscle.
- Energy storage in muscle tissue and liver.
- Glucose polymer, similar to amylopectin, but even
more highly branched.
- A nonlinear polymer of D-glucose units joined
by a-1,4- and a-1,6-glycoside bonds bonds.
- The many branched ends provide a quick means of
putting glucose into the blood.
6
Cellulose
- Polymer of D-glucose, found in plants.
- Mammals lack the ?-glycosidase enzyme.
Average molecular weight of 400,000,corresponds
to approximately 2800 D-glucose units per
molecule.
b-glucosidic linkage
7
Chitin
- Polymer of N-acetylglucosamine.
- Exoskeleton of insects.
8
Polysaccharides Digestion
Glucose Polymers Starch is digestable Cellulose
is not digestable by humans
Why?
Its b
9
Modification of Cellulose
- Cellulose Nitrate
guncotton
- Pyroxylin
Partially nitrated photographic film
- Cellulose Acetate
film
10
Cellulose fibre - Rayon
11
Biological Sugars and reactions
12
Membrane Carbohydrates
- Membranes of animal plasma cells have large
numbers of bound small carbohydrates to them.
- these membrane-bound carbohydrates are part of
the mechanism by which cell types recognize each
other they act as antigenic determinants
- among the first discovered of these antigenic
determinants are the blood group substances
13
ABO Blood Classification
- In the ABO system, individuals are classified
according to four blood types dependent upon
which sugars are present on the surface - A, B, AB, and O
14
Chemstrip Kit
- Blood glucose test for diabetics
- Based on reaction of o-toluidine with glucose
15
Glucose Assay
- Diabetes A common analytical procedure in the
clinical chemistry laboratory is the
determination of glucose in blood, urine, or
other biological fluid
- The o-toluidine test is applied directly to
serum, plasma, cerebrospinal fluid, and urine
samples as small as 20 ?L (microliters) can
be used.
- glucose reacts with 2-methylaniline
(o-toluidine) in the presence of acetic acid
to give an imine which has a blue-green color
- the intensity of the absorption at 625 nm is
proportional to the glucose concentration
- Galactose, mannose, and to a lesser extent
lactose and xylose also react with o-toluidine
to give colored imines and, therefore, have the
potential for false positive.
16
Glucose Assay
- The glucose oxidase method is completely specific
for D-glucose
17
Glucose Assay
- O2 is reduced to hydrogen peroxide H2O2
- the concentration of H2O2 is proportional to the
concentration of glucose in the sample - in one procedure, hydrogen peroxide is used to
oxidize o-toluidine to a colored product, whose
concentration is determined spectrophotometrically
18
Vitamin C - A monosaccharide?
- Vitamin C, vital for life is a necessary part of
our diet because we cannot synthesize it. (Most
plants and animals except primates and guinea
pigs can make their own Vitamin C).
- It is needed to maintain health of dentine,
cartilage, connective tissue and bone.
- Recommended daily allowance 45mg for adults
(60mg if pregnant, 80mg if lactating).
19
Biosynthesis from Glucose
20
Glycocalyx
- The outer viscous covering of fibers extending
from a bacterium
composition The glycocalyx is usually a viscous
polysaccharide and polypeptide slime.
21
Glycocalyx of Intestinal Epithelium Note that
some carbohydrates are covalently attached to
membrane components, while others are secreted as
extracellular matrix Fig 16, The Cell, D.W.
Fawcett (1981)
22
Glycocalyx of Lymphocyte
23
Diagram of Glycocalyx
24
Ribonucleosides
- A ?-D-ribofuranoside bonded to a heterocyclic
base at the anomeric carbon.
25
Ribonucleotides
- Add phosphate at 5 carbon.
26
Nucleic Acids
- Polymer of ribofuranoside rings linked by
phosphate esters.
- Each ribose is bonded to a base.
- Ribonucleic acid (RNA)
- Deoxyribonucleic acid (DNA)
27
Structure of RNA
28
Structure of DNA
- ?-D-2-deoxyribofuranose is the sugar.
- Heterocyclic bases are cytosine, thymine (instead
of uracil), adenine, and guanine.
Linked by phosphate ester groups to form the
primary structure.
29
Base Pairings
30
Double Helix of DNA
- Described by Watson and Crick, 1953.
- Two complementary polynucleotide chains are
coiled into a helix.
31
DNA Replication
32
Other Nucleotides
- Adenosine monophosphate (AMP), a regulatory
hormone.
- Nicotinamide adenine dinucleotide (NAD), a
coenzyme.
- Adenosine triphosphate (ATP), an energy source.