P,T-Flash Calculations - PowerPoint PPT Presentation
1 / 12
Title:
P,T-Flash Calculations
Description:
The system is characterized by T, P and (N-1) mole fractions for each phase ... zi = overall mole fraction of component i. V = vapour phase fraction ... – PowerPoint PPT presentation
Number of Views:231
Avg rating:3.0/5.0
Title: P,T-Flash Calculations
1
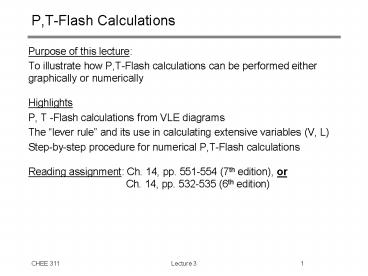
P,T-Flash Calculations
- Purpose of this lecture
- To illustrate how P,T-Flash calculations can be
performed either graphically or numerically - Highlights
- P, T -Flash calculations from VLE diagrams
- The lever rule and its use in calculating
extensive variables (V, L) - Step-by-step procedure for numerical P,T-Flash
calculations - Reading assignment Ch. 14, pp. 551-554 (7th
edition), or - Ch. 14, pp. 532-535 (6th edition)
2
4. P,T-Flash Calculations
- If a stream consists of three components with
widely differing volatility, substantial
separation can be achieved using a simple flash
unit. - Questions often posed
- Given P, T and zi, what are the equilibrium phase
compositions? - Given P, T and the overall composition of the
system, how much of each phase will we collect?
3
P-T Flash Calculations from a Phase Diagram
- For common binary systems, you can often find a
phase diagram in the range of conditions needed. - For example, a Pxy diagram for the
- furan/CCl4 system at 30?C is
- illustrated to the right.
- Given
- T30?C, P 300 mmHg, z1 0.5
- Determine
- x1, x2, y1, y2 and the fraction of the
- system that exists as a vapour (V)
4
Flash Calculations from a Phase Diagram
- Similarly, a Txy diagram can be used if
available. - Consider the ethanol/toluene system illustrated
here at P 1atm. - Given
- T90?C, P 760 mmHg, z1 0.25
- Determine
- x1, x2, y1, y2 and the fraction of the
- system that exists as a liquid (L)
- How about
- T90?C, P 760 mmHg, z1 0.75?
5
Phase Rule for Intensive Variables
- For a system of ? phases and N species, the
degree of freedom is - F 2 - ? N
- variables that must be specified to fix the
intensive state of the system at equilibrium - Phase Rule Variables
- The system is characterized by T, P and (N-1)
mole fractions for each phase - the masses of the phases are not phase-rule
variables, because they do not affect the
intensive state of the system - Requires knowledge of 2 (N-1)? variables
- Phase Rule Equations
- At equilibrium ?i? ?i ? ?i ? for all
N species - These relations provide (?-1)N equations
- The difference is F 2 (N-1)? - (?-1)N
- 2- ? N
6
Duhems Theorem Extensive Properties SVNA10.2
- Duhems Theorem For any closed system of known
composition, the equilibrium state is determined
when any two independent variables are fixed. - If the system is closed and formed from specified
amounts of each species, then we can write - Equilibrium equations for chemical potentials
(?-1)N - Material balance for each species N
- We have a total of ?N equations
- The system is characterized by
- T, P and (N-1) mole fractions for each phase 2
(N-1)? - Masses of each phase ?
- Requires knowledge of 2 N? variables
- Therefore, to completely determine the
equilibrium state we need - 2 N? - ?N 2 variables
- This is the appropriate rule for flash
calculation purposes where the overall system
composition is specified
7
Ensuring you have a two-phase system
- Duhems theorem tells us that if we specify T,P
and zi, then we have sufficient information to
solve a flash calculation. - However, before proceeding with a flash calcn,
we must be sure that two phases exist at this P,T
and the given overall composition z1, z2, z3 - At a given T, the maximum pressure for which two
phases exist is the BUBL P, for which V 0 - At a given T, the minimum pressure for which two
phases exist is the DEW P, for which L 0 - To ensure that two phases exist at this P, T, zi
- Perform a BUBL P using xi zi
- Perform a DEW P using yi zi
8
Ensuring you have a two-phase system
- If we revisit our furan /CCl4 system at 30?C, we
can illustrate this point. - Given
- T30?C, P 300 mmHg, z1 0.25
- Is a flash calculation possible?
- BUBLP, x1 z1 0.25
- DEWP, y1 z1 0.25
- Given
- T30?C, P 300 mmHg, z1 0.75
- Is a flash calculation possible?
- BUBLP, x1 z1 0.75
- DEWP, y1 z1 0.75
9
Flash Calculations from Raoults Law
- Given P,T and zi, calculate the compositions of
the vapour and liquid phases and the phase
fractions without the use of a phase diagram. - Step 1.
- Determine Pisat for each component at T
(Antoines eqn, handbook) - Step 2.
- Ensure that, given the specifications, you have
two phases by calculating DEWP and BUBLP at the
composition, zi. - Step 3.
- Write Raoults Law for each component
- or
- (A)
- where Ki Pisat/P is the partition coefficient
for component i.
10
Flash Calculations from Raoults Law
- Step 4.
- Write overall and component material balances on
a 1 mole basis - Overall
- (B)
- where L liquid phase fraction, V vapour phase
fraction. - Component
- i1,2,,n (C)
- (B) into (C) gives
- which leads to
- (D)
- Step 5.
- Substitute Raoults Law (A) into (D) and
rearrange - (E)
11
Flash Calculations from Raoults Law
- Step 6
- Overall material balance on the vapour phase
- into which (E) is substituted to give the general
flash equation - 14.18
- where,
- zi overall mole fraction of component i
- V vapour phase fraction
- Ki partition coefficient for component i
- Step 7
- Solution procedures vary, but the simplest is
direct trial and error variation of V to satisfy
equation 14.18. - Calculate yis using equation (E) and xis using
equation (A)
12
VLE Calculations Summary
- Here is a summary of what we need to know
(Lectures 8 9) - How to use the Phase Rule (F2-pN)
- How to read VLE charts
- - Identify bubble point and dew point lines
- - Read sat. pressures or temperatures from the
chart - - Determine the state and composition of a
mixture - How to perform Bubble Point, Dew Point, and
P,T-Flash calculations - - Apply Raoults law
- - Apply Antoines equation
- How to use the Lever Rule (graphically or
numerically) - How to construct VLE (Pxy or Txy ) charts for
ideal mixtures