Soil Organic Matter SOM' - PowerPoint PPT Presentation
1 / 14
Title:
Soil Organic Matter SOM'
Description:
Essential oils, natural rubbers, carotenes. Steroids (Solomons, chapter 24, p 974-5) ... Triacylglycerols: Animal fats and vegetable oils. ... – PowerPoint PPT presentation
Number of Views:211
Avg rating:3.0/5.0
Title: Soil Organic Matter SOM'
1
Soil Organic Matter (SOM).
SOM - organic matter in a soil that cannot be
recognized as plant material under a light
microscope. Living soil biota. (Soil
microbiology) The decomposing residues of
plants, animals and microbes. Organic matter
that is resistant to further degradation.
.
.
. .
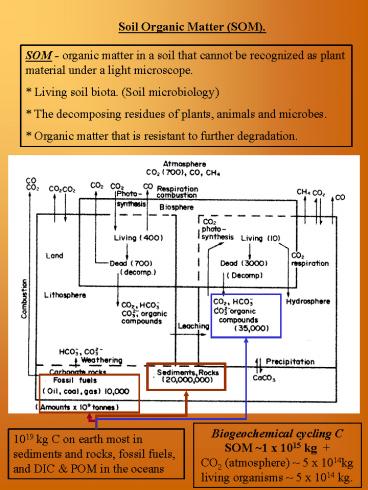
Biogeochemical cycling C SOM 1 x 1015 kg CO2
(atmosphere) 5 x 1014kg living organisms 5 x
1014 kg.
1019 kg C on earth most in sediments and rocks,
fossil fuels, and DIC POM in the oceans
2
Soil Organic Matter (SOM) cont.
At 1 10(vol) C in the soil, 1 hectare (100m x
100m 2.47 acres) to a depth of 15 cm (plough
depth) has about 15 150 tonnes C.
Consider residence times. The most significant
input to SOM is plant residues 11 tonne ha-1
yr-1 for tropical rain forests, TR 1.4 - 14
yr. 6 tonne ha-1 yr-1 for temperate forests, TR
2.5 - 25 yr. 3 tonne ha-1 yr-1 for temperate
grasslands, TR 5 - 50 yr, and lt1 tonne ha-1
yr-1 for deserts, gt15 150 yr. 60 - 70 of
this comes from plant roots (rhizo derived), 70
of plant residues decompose within 1 year. Most
of the C in soils is in the resistate (humic)
form (70).
- Functions of SOM
- Binds particles to form soil aggregates (hinders
soil erosion soils need approximately 4 C to be
structurally stable). - Degrading organics a source of nutrients to soil
- N, P, S, B, metals (Cu, Zn, Mg, Ca, Fe, Mo,
Mn). - Contributes to soil pH.
- Has a high Cation Exchange Capacity -
300meq/100g SOM (21 clays of 100meq/100 g clay,
11 clay 10meq/100g clay). - Controls transport/availability of metals via
complexation adsorption reactions Kf large
for humics, smaller for lower MW carboxylic
acids, amino acids, and organic bases
- (NTA3- as model for complexation).
- Enhances water retention.
- Gives soil a darker colour (heat
adsorption/retention).
3
Soil Organic Matter (SOM) cont.
Soil
Water 20 - 30
Air 20 - 30
Nutrients, trace elements, inorganics
CO2, CH4, H2O
SOM
heat
45 inorganic
0 - 10
Mineralized organics (humics, peat, oils, coal).
SOM
Degrading plant materials (30),
physically and chemically resistant organics
(70)
25 dry matter
Sugars starches - foods. Proteins (10)
Cellulose hemicelluloses -
structural materials. Lipids -
fats/waxes/steroids/etc. (5) Lignins -
structural materials (25)
60
44 C 40 O 8 H 8 ash wt C6H12.8O6
Increasingly resistant to weathering
4
Soil Organic matter (SOM) - Carbohydrates (CH2O)
polyhydroxyaldehydes or ketones or substances
that hydrolyse to give these (Solomons,
Fundimentals of Organic Chemistry, 1994, Chapter
22).
Sugars are mono- or di-saccharides
? D() glucopyranose
? D() glucopyranose
Polymerize to give starch
?oth the hemiacetal OH the C6 CH2OH on the
same face.
Polymerize (dehydration) to give cellulose
? 1 - 4 glycosidic linkages
Long unbranched chains of up to 15,000 glucose
units alternate units turned over
polysacharides. Up to 40 chains held together
by H-bonds to form an insoluble, rigid, fibers
which serve as the structural materials in the
cell walls of plants and some animals. Approximat
ely 50 of C in the biosphere is cellulose. Very
slowly degraded.
5
Soil Organic matter (SOM) - Carbohydrates cont.
? D() glucopyranose
? D() glucopyranose
? 1 - 6 glucosidic linkage
Polymerize to give starch the food reserve in
plants
? 1 - 4 glucosidic linkage
Starch - a major nutrient for animals.
Enzymically assisted digestion involves the
hydrolysis of ? 1 - 4 glycosidic links to produce
oligiosaccharides (a few linked monosaccharides
containing the 1-6 linkages). Further hydrolysis
produces monosaccharides.
6
Soil Organic matter (SOM) - Lignins 25 of SOM
The woody tissues of plants and the major
material binding cells together. Water
repellent. As trees grow it impregnates the
cells and kills them by stopping water and
nutrient transfer across the cell walls.
Highly aromatic polymers (MW - 2000 - 106) based
on phenylpropane monomers.
Many functional groups, acidic, colour, high
complexing capacity.
7
Soil Organic matter (SOM) Proteins. 10 SOM
The most diverse of the biopolymers (starches,
proteins and nucleic acids) Functions
hormones, enzymes, antibodies, haemoglobin,
skin, hair, bone, muscles, tendons, ...
Provide N, S on degradation High molecular
weight polyamides 22 ?-amino acids. MW gt 104.
(Solomons, chapter 24, p 974-5)
- H2O
Soil Organic matter -Lipids 5 SOM
Operationally defined as the compounds of plants
that can be extracted into non-polar solvents
very diverse.
Waxes esters of fatty acids and
alcohols Terpenes molecules based on 2, 3, 4 or
5 isoprene CC(C)CC units. Essential oils,
natural rubbers, carotenes. Steroids
cholesterol
8
Soil Organic matter _ Lipids cont.
Triacylglycerols Animal fats and vegetable
oils. Formed by condensation between glycerol and
various fatty acids (C12 C20). Energy
reserves in animals.
Acyl groups can be saturated, unsaturated or
polyunsaturated. Oils gt 70 unsaturated acyl
groups. Fats lt 40 unsaturated.
Phospholipids One fatty acid replaced by a
phosphate linked to an alcohol - OPO(OH)OR". Eg
of R". -OCH2CH2N(CH3)3 - choline Polar and
non-polar ends sources of P and N on
degradation.
9
Soil Organic matter (SOM) - Nucleic acids
pentose - phosphate backbone
a purine adenine
a pyrimidine base thyamine
guanine
Nucleic acids (Solomons, Chapter 25, p1017)
polymers of nucleotides (phosphate, ribose or
deoxyribose and a purine or a pyrimidine base.
Degrade to a base, phosphate and a sugar.
Adenosine triphosphate (ATP) energy transmitting
molecules. Lose PO43- to give ADP and AMP and
energy for biomolecule fromation.
10
Soil Organic Matter - The Resistant Fraction.
- 70 of SOM is the chemically physically
resistant organics. - Derived in some way from the plant residue inputs
? - similar structural units to the biomolecules,
- similar functional groups but not readily
hydrolysed or oxidised. - not clearly structurally defined (nor
properties). Depend on inputs and location
there is no one resistant SOM. - Main contributor to many SOM properties
- CEC (150 - 300 meq/100g), complexing capacity,
soil pH, - water retention ( 80 of its own weight), colour
(brown/black).
A suggested structure.
Acidic phenolic functional groups
colour
Clay particle
Protein residue
Carbohydrate residue
Fe(OH)3 coating
Lignin residue
colour
Has a high MW (gt103), strongly adsorbed to
particle surfaces, highly oxidised, extensively
conjugated.
11
The Characterization of Humic Materials
a) Isolation. Operationally defined by
isolation procedure (cf lipids).
2. 0.5M NaOH 24 - 48 hrs, N2 atm, 10g soil/dm-3
caustic
1. HCl CO3- ?CO2
3. Centrifuge
Soil
a insoluble HUMIN
Soluble phase
Elemental analyses (C, H, N, S O by
difference) on an ash free, dry weight basis
mole wt mole
ratio C 54 4.5 1.7 H 4
4 1.5 O 42 2.6
1 N, S traces
1. 0.5M HCl 24hrs.
2. centrifuge
Soluble Fulvic Acid
Insoluble Humic Acid
unsaturated high O but insoluble ?
ether, but not acid or phenol groups.
yellow/red solution purify on a cation
exchange resin
dark red/brown solid purify by -
recrystallization (NaOH and HCl) - dialysis
against water (removes cations) speciate using
MW cutoff dialysis.
mole wt mole
ratio C 45 3.8 1.4 H 5
5 1.8 O 45 2.8 1 N
2 0.14 S 2 0.06
C14H18O10
mole wt
mole ratio C 55 4.6 2.1 H
5 5 2.3 O 35 2.2
1 N 3 0.2 S 1
0.03 C21H23O10
CH 11 ? high degree of unsaturation
more O more saturated
12
The Characterization of Humic Materials cont.
b) Acidity The phenolic, R-OH and R-COOH
functional groups give acidity but the many
functional groups in many chemical environments
prohibit the definition of an acid dissociation
constant - many pKas but they will be
experimentally indistinguishable. Therefore
titrate with standard base over a defined pH
range - operationally defined. FA 5meq/g.
For HA dissolve in excess base and back titrate
with standard acid.
Al(OH)3 OH- ? Al(OH)4- Gibbsite dissolution
under Bayer conditions (3.5M NaOH, 140C)
13
The Characterization of Humic Materials cont.
c) Vibrational spectroscopy
(C-C)aromatic stretching
(CO)acyl stretching
OH stretch
(C-H)aliphatic stretching
Kingston Harbour sediment Humic Acid
C-O stretching C-OH bending
Kingston Harbour sediment Fulvic Acid
14
The Characterization of Humic Materials cont.
d) NMR spectroscopy (Solomons Chapter 14)
13C, solid state or NaOH solution.
Ho Happlied(1-?) where ? is a shielding
constant. The observed field at the nucleus is
shifted from the applied field by magnetic
properties of the shielding electrons. Quote the
field at which resonance occurs relative to a
standard (usually tetramethylsilane - TMS).
Broad band spectra (materials not homogeneous,
solid state NMR have complicated orientational
effects and lattice-nuclear spin interactions).
Chemical shifts indicate functional groups.
areas under peaks indicate relative amounts of
functional groups.