Chapter 11: Theres really no problems only Solutions - PowerPoint PPT Presentation
1 / 16
Title:
Chapter 11: Theres really no problems only Solutions
Description:
(P = kC in study guide, either is OK, the k values will be the inverse of each other) ... If both were solutions but the one on the left had a lower concentration of ... – PowerPoint PPT presentation
Number of Views:95
Avg rating:3.0/5.0
Title: Chapter 11: Theres really no problems only Solutions
1
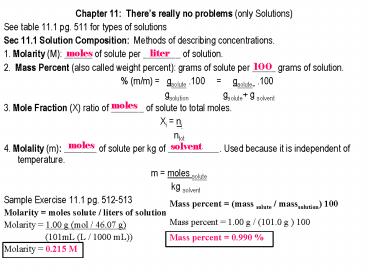
- Chapter 11 Theres really no problems (only
Solutions) - See table 11.1 pg. 511 for types of solutions
- Sec 11.1 Solution Composition Methods of
describing concentrations. - 1. Molarity (M) ______ of solute per ________ of
solution. - 2. Mass Percent (also called weight percent)
grams of solute per _____ grams of solution. - (m/m) gsolute .100
gsolute .100 -
gsolution gsolute g solvent - 3. Mole Fraction (X) ratio of _______ of solute
to total moles. - ?i ni
- ntot
- 4. Molality (m) _______ of solute per kg of
___________. Used because it is independent of
temperature. - m moles solute
- kg solvent
- Sample Exercise 11.1 pg. 512-513
- Molarity moles solute / liters of solution
- Molarity 1.00 g (mol / 46.07 g)
- (101mL (L / 1000 mL))
- Molarity 0.215 M
moles liter
100
moles
moles
solvent
Mass percent (mass solute / masssolution) 100
Mass percent 1.00 g / (101.0 g ) 100
Mass percent 0.990
2
- Mole fraction (moles solute / moles solution)
? 1.00 g (mol / 46.07 g) (1.00
g (mol / 46.07 g C2H5OH ) 100.0 g (mol / 18.015
g H2O))
? (0.021706 moles) (0.021706
moles C2H5OH 5.5509 g H2O)
? 0.021706 moles 5.5726 moles
? 0.00390
Molality moles solute / kg solvent
Molality 1.00 g (mol / 46.07 g)
(100. g (kg / 1000 g))
Molality 0.217 m
3
- 5. Normality (N) equivalents of solute per liter
of solution. See table 11.2 pg. 513 - a. for acids multiply the molarity by the number
of ionizable ___ ions. - b. for bases multiply the molarity by the number
of ionizable ____ ions. - c. Redox reactions multiply the molarity the
number of ____ consumed by the reaction. - For each of these Zumdahl will determine the
equivalent molar mass from the quotient of the
molar mass and the number of equivalents (H, OH-
or e-) transferred by the reaction. - Sample Exercise 11.2 pg. 515
H
OH-
e-
Mass percent (mass solute / mass solution) 100
( ) 100
Mass percent 3.75 moles H2SO4 98.086 g
H2SO4 ml solution
1000 ml solution mole H2SO4 1.230 g
solution
Mass percent 29.9
molality (moles solute / mass solvent)
molality 29.904 g H2SO4 moleH2SO4
1000 g (100 -
29.904) g H2O 98.086 g H2SO4 kg
molality 4.35 m
Normality 3.75 M (2) 7.50 N
4
- Try this An aqueous solution has a solute
concentration of 25.0 by mass. The density of
the solution is 1.20 g/cm3. Determine the
molality, molarity, and mole fraction of the
solute in the solution.The molar mass of the
solute is 80.0 g/mol.
molality moles solute / kg solvent
molality 25.0 g solute mole solute
1000 g solvent 4.17 m 75.0 g
solvent 80.0 g solute kg solvent
\
\
\
\
Molarity moles solute / L solution
Molarity 25.0 g solute mole solute
1.20 g solution 1000 mL solution
100 g solution 80.0 g solute mL
solution L solution
\
\
\
\
\
\
Molarity 3.75 M
? moles solute / (moles solute moles solvent)
? (25.0 g (mol / 80.0 g)) (25.0 g (mol / 80.0
g)) 75.0 g (mol / 18.015 g))
? 0.3125 mol (0.3125 mol 4.1632 mol)
Homework Day 1 pg. 547-548 9 11 13 27
(for Sulfuric acid only) 29 Day 2 pg. 547-548
10 12a-d 26 30-32 (hint on 30 the solvent
is water whose density is 1.00 g/ml)
? 0.3125 mol (4.4757 mol)
? 0.0698
5
- Sec 11.2 The Energies of Solution Formation
- When determining if a solute (like sugar) will
dissolve in a solvent (like water) the general
rule like dissolves like is utilized. A polar
solute will dissolve in a __________ solvent
where a non-polar solute will dissolve in a
non-polar solvent. - Whether a solute dissolves or not is determined
by the free energy of solution formation. Free
energy is a combination enthalpy (exothermicity
or endothermicity )and entropy (tendency towards
disorder). - ?G ?H - T?S (for a spontaneous process ?G is
negative) - When dealing with the enthalpy, we will consider
3 steps of solution formation. - Step 1 enthalpy to separate solute particles
(always positive) - Step 2 enthalpy to separate solvent particles
(always positive) - Step 3 enthalpy of mixing solvent and solute
particles (can be positive (like water and oil) - or negative(sugar and water))
- See fig. 11.1 pg. 517
- The combination of steps 2 and 3 is referred to
as the enthalpy of hydration. - See table 11.3 pg. 518
- Sample exercise 11.3 pg. 518
- Micelles aid in the dissolving of non-polar
solutes in polar solvents. Micelles formed from
molecules with a polar head and a long non-polar
tail (soaps and detergents create micelles in oil
water mixtures create micelles). - Sec 11.3 Factors Affecting Solubility
- 1. Structure of non-polar or polar solutes and
solvents. For example Vitamins have variable
solubilities. Vitamins A,B,E and K are fat
soluble due to being __________ (called
hydrophobic water fearing) while vitamin C is
water soluble due to being ________ (called
hydrophilic water loving).
polar
nonpolar
polar
6
- 2. Pressure acting on a gas can greatly increase
its solubility in liquids (for example carbonated
beverages). The solubility is governed by Henrys
Law - C kP (P - pressure, k Henrys Law constant, C
concentration) - (P kC in study guide, either is OK, the k
values will be the inverse of each other) - This equation only works for slightly soluble
gases and will not work for gases that react or
ionize in the solvent (such as water in HCl).
See fig. 11.5 pg. 521. - Sample Exercise 11.4 pg. 522
- 3. Temperature Effects
- a. An increase in temperature may increase or
decrease the solubility of salt in water (fig.
11.6 pg. 522). - b. An increase in temperature will _____________
the solubility of a gas in water. (fig. 11.7 pg.
524) - Homework
- pg. 547-549 14-16 33 35-39 41 43 (dont
have to determine k) Bonus 44
C kP
C kP
C (3.1.10-2 M/atm) (5.0 atm)
C (3.1.10-2 M/atm) (4.0.10-4 atm)
C 0.16 M
C 1.2.10-5 M
decrease
7
Exercise 11. 5,6
Sec 11.4 The Vapor Pressure of Solutions (Example
s in Power Point) 1. Liquid solutions have
different properties than _______ solvents
(antifreeze. Salt on icy roads). 2. The vapor
pressure of a solution containing a nonvolatile
solute is much ________ than the vapor pressure
of a pure solvent (fig. 11.10) 3. See fig. 11.9
to observe the migration of particles from the
higher vapor pressure pure water to the solution.
If both were solutions but the one on the left
had a lower concentration of solute, particles
would still migrate to the container on the right
until the concentrations of the solutions were
__________ 4. We can conclude that the addition
of a nonvolatile solute to a solvent will
________ the vapor pressure. Raoults Law
Psolution Xsolvent Posolvent (for pure
solvent X 1) See fig. 11.11 pg. 526
pure
lower
equal
lower
- Psoln Xsolvent Posolvent
- Determine moles of solvent and solute
- mole H2O 643.5 cm3(0.9971 g / cm3)(mol /
18.015 g) mole sucrose 158.0 g (mol sucrose
/342.3 g) - 35.6166 mol H2O 0.46158 mol sucrose
- Xsolvent 35.6166 mol H2O / (35.6166 mol
0.46158 mol) - 35.6166 mol H2O / 36.0782
mol - 0.98721
- Psoln 0.98721 (23.76 torr)
- Psoln 23.46 torr
8
Exercise 11.6
- Psoln Xsolvent Posolvent
- Determine moles of solvent and solute
particles - mole H2O 175 g H2O (mol / 18.015 g)
9.7141 mol H2O - mole particles 35.0 g Na2SO4 (mol /142.04 g)(3
mol particles / 1 mol Na2SO4) 0.73923 mol
particles - Xsolvent 9.7141 mol H2O / (9.7141 mol 0.73923
mol) 9.7141 mol H2O / 10.4533 mol 0.92928 - Psoln 0.929729 (23.76 torr)
- Psoln 22.1 torr
- 5. Some solutions contain volatile solutes which
may contribute to the _______ pressure of the
solution - (unlike those discussed above). Fig. 11.12 pg.
529 - 6. Raoults Law must be modified to account for
the vapor phase contributions of solute. - Ptotal XAPoA XB PoB
- 7. The mole fraction of solute or solvent in the
vapor phase is governed by PA XA Ptot - (where Ptot XAPoA XB PoB and PA XAPoA so
XA XAPoA / (XAPoA XB PoB) - Nonideal Solutions
- 1. Not all solutions obey Raoults law and are
called ________________ solutions. - 2. Ideal solutions arise when solute / solvent
interactions are nearly _____________. - When ?H of solution formation is __ the solute
and solvent particles interact in nearly
identical fashions - and there is no deviation to Raoults law.
see fig. 11.13 A - b. When ?H of solution formation is __
(endothermic) a positive deviation to Raoults
Law is observed - (particles are not interacting) see fig.
11.13 b - c. When ?H of solution formation is __
(exothermic) a negative deviation to Raoults Law
is observed - (particles are interacting strongly) see
fig. 11.13 c - Table 11.4 pg. 530.
vapor
non-ideal
identical
0
-
9
Exercise 11.7
- Ptotal XAPoA XBPoB (vapor pressure from
solution) - Each X is 0.500
- Ptotal 0.500 (345 torr) 0.500 (293 torr)
- Ptotal 172.5 torr 146.5 torr
- Ptotal 319 torr
- 319 torr is higher than the observed vapor
pressure of 260 torr, this would be a negative
deviation due to dipole dipole interactions. - What is the Vapor Phase mole Fraction?
- PA XA Ptot
- XA PA / Ptot
- XA XAPoA / (XAPoA XBPoB)
- XA 172.5 torr / 319.0 torr
- XA 0.541 XB 1 XA 0.459
Homework Composition means mole fraction Day
1 pg. 547-550 s 18 19 45 49 51 53
56 Day 2 pg. 549-550 s 46 47 48 50 54
55 Bonus 52
XA, XB XA,XB
10
- Colligative Properties properties that depend
upon the _________ of solute particles in
solution. - Sec 11.5 Boiling Point Elevation and Freezing
Point Depression - 1. Normal boiling occurs when the vapor pressure
of a liquid is _______ to atmospheric pressure. - 2. The presence of a nonvolatile solute _________
the vapor pressure and will thereby _________ the
boiling point (The presence of a non-volatile
solute will lower the melting point). fig. 11.14
pg 532 - 3. The magnitude of the elevation depends upon
the concentration of solute (molality is utilized
because it is independent of _________________).
- ?tb i kbm solute ?tf i kfm solute
- i number of particles per solute particle (i
is called the vant Hoff factor) - for covalent compounds i __ for NaCl i __
for Na3PO4 i __ see table 11.5 for values of kb
and kf - 4. As an ionic compound solution becomes more
concentrated, i deviates from its ideal value due
to _________ of the ions. Therefore (for more
concentrated solutions) i will appear lower than
its ideal value. - See fig. 11.22 pg. 532 and table 11.5
number
equal
lowers
raising
temperature
4
1
2
pairing
11
- Sample 11.8
- Molar Mass grams / mole
- Moles can be determined from molality
- ?tb i kb m solute
- m solute ?tb / (i kb)
- m solute 0.34oC / (1(0.51oC/m))
- m solute 0.6667 m
- ? Moles 0.1500 kgsolvent (0.6667 molsolute /
kgsolvent) - 0.1000 mol
- Molar Mass 18.0 g / 0.1000 mol
- Molar mass 180 g /mol
12
- Sample Exercise 11.9
- ?tf i kf m solute
- m solute ?tf / (i kf)
- m solute 23.3oC / (1(1.86oC/m))
- m solute 12.527 m
- ?g 10.0 kg solvent (12.527 mol /
kgsolvent)(62.1 g/mol) - 7.78.103 g
13
- Sample 11.10
- Molar Mass grams / mole
- Moles can be determined from molality
- ?tf i kf m solute
- m solute ?tf / (i kf)
- m solute 0.240oC / (1(5.12oC/m))
- m solute 0.046875 m
- ? Moles 0.0150 kgsolvent (0.046875 molsolute /
kgsolvent) - 0.000703125 mol
- Molar Mass 0.546 g / 0.000703125 mol
- Molar mass 776 g /mol
Homework Day 1 pg. 546-551 (5-6 dont have to
write) 57 59 61 63 71a also The freezing
point depression of a 0.091 m solution of CsCl is
0.302oC. The freezing point depression of a
0.091 m solution of CaCl2 is 0.440oC. In which
solution does ion association appear to be
greater? use ionization ((iactual 1 ) /
(itheoretcial - 1)). 100 to determine your
answer. Day 2 pg. 547-551 (20, 69 dont have
to write) 58 60 62 64 73 83
14
- Sec 11.6 Osmotic Pressure See figs 11.16-11.18
pg. 536 - 1. When a semipermeable membrane (allows passage
of solvent but not _________ particles) is placed
between a solution and pure solvent, pure solvent
will travel into the solution. This flow of
solvent is referred to as _____________. - 2. Solvent will flow into the solution until a
pressure develops such that the rate of transfer
is _________ in and out of the solution. - 3. This hydrostatic pressure is called the
_____________ pressure. - 4. Osmosis can be prevented by application of
______________ to the solution. The minimum
pressure that will stop osmotic flow is equal to
the osmotic pressure. - 5. Osmotic pressure (?) is calculated as follows
- ? i CRT
- (utilize 0.08206 L atm /mol K and K temperature
C n / V) - 6. Pressures applied to the solution that are
greater than the osmotic pressure will cause
solvent to travel out of the solution (this is
called ______________ osmosis). fig. 11.20 pg.
539 Reverse osmosis is utilized in the
desalinization of water (see fig. 11.21 pg. 540). - Sample Exercise 11.11 and 11.12 pg. 537-538
solute
osmosis
equal
osmotic
pressure
reverse
C ? / (iRT)
n/V ? / (iRT)
Molar mass mass / moles
Molar mass 1.00.10-3 g / moles
n V(? / (iRT))
n 1.00.10-3 L (1.12 torr (1 atm/760 torr))
1(0.08206 Latm / mol K)(298K)
Molar mass 1.00.10-3 g / 6.0264 .10-8 mol
Molar mass 1.66.104 g/mol
n 6.0264.10-8 mol
15
- Sec 11.12
- ? iCRT
- C ? /(iRT)
- C (7.70 atm/2(0.08206 Latm/molK)(298 K))
- C 0. 157 M
- Dialysis
- 1. Osmosis allows the passage of _______ solute
particles while dialysis allows the flow of both
solute and solvent particles. - 2. Most important application of dialysis is the
use of an artificial kidney to remove waste from
human ______. The persons blood flows through a
semipermeable cellophane tube that is immersed in
a solution that has the _________ concentration
of ions and particles as blood but has none of
the waste particles. See fig. 11.19 pg. 538 - 3a. Solutions of identical osmotic pressure are
called ______________ solutions. IV solutions
are isotonic with solutions within the body. - b. Cells bathed in hypertonic solutions (more
concentrated in solute than the cells) will
__________ due to flow of solvent from the cells
in a process referred to as crenation. This is
why sea water is not drinkable. - c. Cells bathed in hypotonic solutions (less
concentrated in solute than the cells) will
__________ due to flow of solvent into the cells
in a process referred to as hemolysis.
11.13 expected i 5
Experimental i ?/CRT
i (10.8 atm) 0.10 M(0.08206 Latm/ mol K)(298
K) i 4.4
There must be some ion pairing
small
blood
same
isotonic
shrivel
explode
16
- Colloids See table 11.7 pg. 543
- 1. Colloids are a kind of mixture in which the
solute particles are so _______ that the do not
settle as suspensions do (the particles due not
settle due to electrostatic repulsions fig. 11.24
pg. 544). - 2. The particles are large enough to ___________
light (solution particles do not scatter light)
to exhibit what is called the Tyndall effect.
fig. 11.23 pg. 543 - 3. The size range of solute particles for
colloids is from 1 to 1000 nm. - 4. The destruction of a colloid is called
coagulation and may be achieved by ______________
or adding an electrolyte to the colloid. Removing
soot from smoke is an example of coagulation
(fig. 11.25 pg. 545).
small
scatter
heating
Homework pg. 547551 (dont have to write 21
22 84) 65 66 67 68 70 72 Also Po of H2O
at 29.6oC is 31.1 torr. 86.7 g of a nonvolatile
covalent compound is added to 350.0 g of water to
give a solution whose vapor pressure is 28.6
torr. Determine the molar mass of the compound.