An Ice Cream Sundae Analogy for Limiting Reactions - PowerPoint PPT Presentation
1 / 28
Title:
An Ice Cream Sundae Analogy for Limiting Reactions
Description:
An Ice Cream Sundae Analogy. for Limiting Reactions. Fig. 3.10. Practice Problem 3.85 ... O2 0.7291 mol SO2. CS2 0.7879 mol SO2. O2 is limiting 46.7 g SO2. 2.23 ... – PowerPoint PPT presentation
Number of Views:21
Avg rating:3.0/5.0
Title: An Ice Cream Sundae Analogy for Limiting Reactions
1
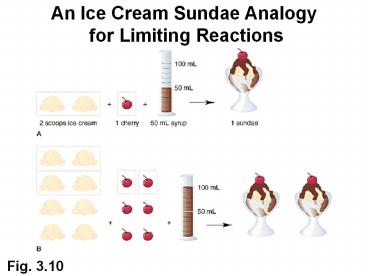
An Ice Cream Sundae Analogy for Limiting
Reactions
Fig. 3.10
2
Practice Problem 3.85
3
Practice Problem 3.86
4
O2? 0.7291 mol SO2
O2 is limiting ? 46.7 g SO2
CS2 ? 0.7879 mol SO2
2.23 g CS2 remaining
5
Chapter 4 Chemical Reactions An Introduction
6
Suggested Problems for Chapter 4
4.9, 4.12, 4.17, 4.18, 4.23, 4.25, 4.28, 4.29,
4.32, 4.37, 4.39, 4.44, 4.47, 4.55, 4.59, 4.65.
4.73, 4.75, 4.79, 4.95, 4.97, 4.131
7
Figure 4.1 Reaction of potassium iodide solution
and lead (II) nitrate solution. Photo courtesy of
James Scherer.
8
Electrolytes
9
Figure 4.2 Motion of ions in solution.
10
Figure 4.3 Testing the electrical conductivity
of a solution water.Photo courtesy of American
Color.
11
Figure 4.3 Testing the electrical conductivity
of a solution sodium chloride.Photo courtesy of
American Color.
12
Figure 4.4 Comparing strong and weak
electrolytes HCl. Photo courtesy of American
Color.
13
Figure 4.4 Comparing strong and weak
electrolytes NH3. Photo courtesy of American
Color.
14
Methanol
Li
15
The Role of Water as a Solvent The Solubility
of Ionic Compounds
Electrical conductivity - The flow of electrical
current in a solution is a
measure of the solubility of ionic
compounds or a
measurement of the presence of ions in
solution. Electrolyte - A substance that
conducts a current when dissolved in
water. Soluble ionic compound
dissociate completely and
may conduct a large current, and are called
strong Eeectrolytes.
NaCl(s) H2O(l)
Na(aq) Cl -(aq)
When sodium chloride dissolves into water the
ions become solvated, and are surrounded by water
molecules. These ions are called aqueous and
are free to move through out the solution, and
are conducting electricity, or helping electrons
to move through out the solution
16
Fig. 4.2
17
(No Transcript)
18
The Solubility of Ionic Compounds in Water
The solubility of ionic compounds in water
depends upon the relative strengths of the
electrostatic forces between ions in the ionic
compound and the attractive forces between the
ions and water molecules in the solvent. There
is a tremendous range in the solubility of ionic
compounds in water! The solubility of so called
insoluble compounds may be several orders of
magnitude less than ones that are
called soluble in water, for example
Solubility of NaCl in water at 20oC 365
g/L Solubility of MgCl2 in water at 20oC 542.5
g/L Solubility of AlCl3 in water at 20oC 699
g/L Solubility of PbCl2 in water at 20oC 9.9
g/L Solubility of AgCl in water at 20oC 0.009
g/L Solubility of CuCl in water at 20oC 0.0062
g/L
19
(No Transcript)
20
Types of Chemical Reactions
- Most reactions fall under three basic types
- 1) Precipitation Reactions
- 2) Acid-Base Reactions
- 3) Oxidation-Reduction Reactions (RedOx)
21
Precipitation
22
Solubility
- Soluble ability to dissolve in a liquid
- Insoluble inability to dissolve in a liquid
- Not all Ionic Compounds are water soluble
- Not all molecular compounds are insoluble!
23
Reactions Involving IonsMolecular vs. Ionic
Equations
- Chemical Reaction can be expressed by
- Molecular Equation (balanced chemical equation)
- Complete Ionic Equation (showing all ions in
reaction) - Net Ionic Equation (showing only those ions
directly involved in reaction) - Consider
- Copper (III) sulfate reacts with sodium hydroxide
to form copper (III) hydroxide and sodium sulfate
(all in water).
- Express reaction in molecular, complete ionic,
- and net ionic equations
24
8 Simple Rules For Common Ionic Compounds
25
Song For Solubility!!
(Taken from Cornell University Adapted by Daley
Sing to Rhythm of 99 Bottles)
Potassium, sodium, and ammonium salts,
Whatever they may be, Can always be depended
on For solubility. Asked about the nitrates
or acetates The answer is always clear, They
each and all are soluble, Is all we want to
hear. Most every chloride's soluble At least
we've always read Save silver, mercurous
mercury And (slightly) chloride of lead. Take
the Bromide and iodide salts There soluble as
can be Save silver, mercury, and lead That
precipitate as you see Every single sulfate Is
soluble , 'Tis said 'Cept barium and
strontium And calcium and lead.
Hydroxides of metals won't dissolve That is, all
but three Potassium, sodium and
ammonium Dissolve quite readily. And then you
must remember That you must not
"forgit" Calcium, barium, strontium Dissolve a
little bit. The carbonates are insoluble,
It's lucky that it's so, Or else, our marble
buildings Would melt away like snow. (Repeat
with feeling) Only note is that all Lithium
salts are Soluble too!!!
26
Predicting Whether a Precipitation Reaction
Occurs Writing Equations
a) Calcium Nitrate and Sodium Sulfate solutions
are added together.
Molecular Equation
Ca(NO3)2 (aq) Na2SO4 (aq)
CaSO4 (s) NaNO3 (aq)
Total Ionic Equation
Ca2(aq)2 NO3-(aq) 2 Na(aq) SO4-2(aq)
CaSO4 (s) 2 Na(aq) 2 NO3-(aq)
Net Ionic Equation
Ca2(aq) SO-4(aq)
CaSO4 (s)
Spectator Ions are Na and NO3-
b) Ammonium Sulfate and Magnesium Chloride are
added together.
In exchanging ions, no precipitates will be
formed, so there will be no chemical reactions
occurring! All ions are spectator ions!
27
Precipitation Reactions Will a Precipitate Form?
If we add a solution containing potassium
chloride to a solution containing ammonium
nitrate, will we get a precipitate?
KCl(aq) NH4NO3 (aq) K(aq)
Cl-(aq) NH4(aq) NO3-(aq)
By exchanging cations and anions we see that we
could have potassium chloride and ammonium
nitrate, or potassium nitrate and
ammonium chloride. In looking at the solubility
table it shows all possible products as soluble,
so there is no net reaction!
KCl(aq) NH4NO3 (aq) No Reaction!
If we mix a solution of sodium sulfate with a
solution of barium nitrate, will we get a
precipitate? From the solubility table it shows
that barium sulfate is insoluble, therefore we
will get a precipitate!
Na2SO4 (aq) Ba(NO3)2 (aq)
BaSO4 (s) 2 NaNO3 (aq)
28
(No Transcript)