Principles of Bioinorganic Chemistry - 2003 - PowerPoint PPT Presentation
Title:
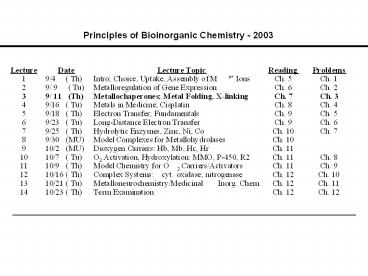
Principles of Bioinorganic Chemistry - 2003
Description:
Principles of Bioinorganic Chemistry 2003 – PowerPoint PPT presentation
Number of Views:201
Avg rating:3.0/5.0
Title: Principles of Bioinorganic Chemistry - 2003
1
Principles of Bioinorganic Chemistry - 2003
2
Metalloregulation of Iron Uptake and Storage
Bacteria A single protein, Fur (for iron uptake
regulator), controls the transcription of genes
involved in siderophore biosynthesis. Fur is a
dimer with subunits of Mr 17 kDa. At high iron
levels, the Fur protein has bound metal and
interacts specifically with DNA repressing
transcription. Mammals Expression of ferritin
and the transferrin receptor is regulated at the
translational level.
3
Components of the Metalloregulatory System
Fe
IRP
Iron-responsive protein (IRP)
Stem-loop structure in the mRNA
IRP
4
Regulation events High Fe, low TfR, high Ft Low
Fe, high TfR, low Ft
Fe
IRP
Message translated
Message degraded
Ferritin
Transferrin
IRP
Message blocked
Message translated
5
IRP1 is the Cytosolic Aconitase Contains an Fe4S4
Cluster
Cluster assembled in protein, which then
dissociates from mRNA
Apoprotein stays associated with mRNA
6
Metallochaperones Metal Folding
PRINCIPLES
- Metallochaperones guide and protect metals to
natural sites - Chaperone and target receptor protein
structurally homologous - Metal-mediated protein structure changes affect
transcription - Metal-mediated protein structure changes affect
translation - Metal-induced protein structure changes also
activate enzymes - Metal-induced bending of DNA affects function
- Metal ionic radii and ML water bridging are used
to advantage
ILLUSTRATIONS
- Copper insertion into metalloenzymes
- Zinc finger proteins control transcription
- Ca2, a second messenger and sentinel at the
synapse - Cisplatin, an anticancer drug
7
2O2- 2H H2O2 O2
8
Copper Uptake and Transport in Cells
The players SOD, superoxide dismutase, a copper
enzyme, a dimer containing two His-bridged
Cu/Zn sites CCS, a copper chaperone for
superoxide dismutase Lys7, the gene encoding
yCCS in yeast CCS and SOD1 co-localize in
human tissue Ctr, family of membrane proteins
that transport copper across the plasma
membrane, delivering it to at least three
chaperones CCS, Cox17, Atx1 The puzzles The
total cellular Cu in yeast is 0.07 mM, none
free How does copper find its way into
metalloproteins? The implications Mn, Fe, Zn
have similar systems understanding one in
detail has implications for all
9
Two well characterized pathways
Atx1 delivers Cu to transport ATPases in the
secretory pathway, which translocates it into
vesicles for insertion into multicopper oxidases
such as ceruloplasmin Mutations in human forms
of these ATPases lead to Menkes and Wilson
diseases CCS delivers copper to Cu,Zn SOD Human
Cu,Zn SOD is linked to ALS
10
Key Questions Address by Structural
Bioinorganic Chemistry (Rosenzweig, OHalloran,
Culotta)
What are the details of copper binding by
these proteins, including stoichiometry and
coordination geometry?
How do these chaperones interact with their
copper receptor proteins?
What features of the copper binding and
protein-protein interactions render each
chaperone specific for its target protein?
11
Structure of the Hg(II) form of Atx1
Cys 15
Hg
Hg(II) is exposed at the surface of the protein,
which is reasonable for a protein that functions
in metal delivery-- metal sites in enzymes are
more buried. Hg(II) coordinated by the 2
cysteines. The apo protein has same structure
but with a disulfide bonds between the
cysteine residues.
Cys 18
C
N
12
More Details of the 1.2Å Structure, Active Site
Cys 15
Thr 14
Ser 16
Val 12
2.33 Å
Hg
2.34 Å
Ser 19
Met 13
Cys 18
Lys 65
Ala 21
13
Structure of the Cu Hah1 Protein, the Human
Homolog
C
N
First copper chaperone structure with Cu
bound The two molecules are primarily held
together by the bound metal ion and some hydrogen
bonding
14
Extended H-Bonding Interactions Stabilize the
Structure
T11B is conserved in most related domains. When
it is not there it is replaced by His,
which could serve the same function.
15
Postulated Mechanism for Metallochaperone Handoff
of Copper to a Receptor Protein (OHalloran,
Rosenzweig, Culotta, 2000)
HgAtx1
HgHah1
CuHah1
AgMenkes4
16
N
yCCS1 Crystal Structure
- Domain I (Atx1-like)
- metal binding
- not essential
- Domain II (SOD1-like)
- target recognition
C
C20
C17
- Domain III
- metal delivery
- crucial
Lamb, et al. Nature Struct. Biol. 1999, 6, 724-729
17
Dimer of Dimers Model
54 kDa
32 kDa
86 kDa
- SOD1 homodimer is very stable
- yCCS and hCCS are dimeric in the crystal and
- in solution (yCCS under some conditions)
18
Heterodimer Model
43 kDa
32 kDa
54 kDa
- Structures indicate heterodimer formation is
feasible - Heterodimer formation between different SOD1s
- has been observed
19
Biophysical and biochemical studies of complex
formation
- According to gel filtration chromatography,
dynamic light - scattering, analytical ultracentrifugation,
and chemical - crosslinking experiments, yCCS and SOD1 form a
specific - protein-protein complex
- The molecular weight of the complex, 43 kDa, is
most - consistent with a heterodimer
- Higher order complexes, such as a dimer of
dimers, were - not detected
43 kDa
86 kDa
Lamb, et al. Biochem. 2000, 39, 14720-14727
20
Factors Affecting Heterodimer Formation
- The heterodimeric complex formed with a mutant of
SOD1 - that cannot bind copper, H48F-SOD1, is more
stable - Heterodimer formation is facilitated by zinc
- Heterodimer formation is apparently independent
of whether - copper is bound to yCCS
- Heterodimer formation between Cu-yCCS and wtSOD1
in - the presence of zinc is accompanied by SOD1
activation - These data suggest that in vivo copper loading
occurs via a - heterodimeric intermediate
Lamb, et al. Biochem. 2000, 39, 14720-14727
21
Crystals of the yCCS/H48F-SOD1 heterodimeric
complex
P3221 a b 104.1 Å, c 233.7 Å Solved by
molecular replacement
Lamb, et al. Nature Struct. Biol. 2001, in press.
22
Domain I
SOD1 homodimer
yCCS homodimer
Domain III
Domain II
H48F-SOD1 monomer
yCCS monomer
23
Domain I
Domain II
Domain III
24
Two heterodimers in the asymmetric unit
Loop 7
Loop 7
25
Domain II
Domain I
C20
C17
C20
C229
C17
Domain III
C231
26
C146
S-S subloop
C57
C57
SO42-
27
C146
F48
C57
C229
C231
28
Mechanism of metal ion transfer
- yCCS Domain I probably does
- not directly deliver the metal ion
- yCCS Domain III is well positioned
- in the heterodimer to insert the
- metal ion
- Transient intermonomer disulfide
- formation may play a role in yCCS
- function
His 63
His 48
His 46
His 120
Cys 57
Cys 229
Cys 231
29
Metallochaperones Metal Folding
PRINCIPLES
- Metallochaperones guide and protect metals to
natural sites - Chaperone and target receptor protein
structurally homologous - Metal-mediated protein structure changes affect
transcription - Metal-mediated protein structure changes affect
translation - Metal-induced protein structure changes also
activate enzymes - Metal-induced bending of DNA affects function
- Metal ionic radii and ML water bridging are used
to advantage
ILLUSTRATIONS
- Copper insertion into metalloenzymes
- Zinc finger proteins control transcription
- Ca2, a second messenger and sentinel at the
synapse - Cisplatin, an anticancer drug
30
Zinc Fingers - Discovery, Structures
A. Klug, sequence gazing, proposed zinc fingers
for TFIIIA, which controls the transcription of
5S ribosomal RNA. Zn2 not removed by EDTA. 9
tandem repeats. 7-11 Zn/protein.
Y or F X C X2,4 C X3 F X5 L X2
H X3,4 H X2,6
C
C
C
H
H
H
H
H
The coordination of two S and 2 N atoms from Cys
and His residues was supported by EXAFS ZnS,
2.3 Å ZnN, 2.0 Å. Td geometry. The protein
folds only when zinc is bound gt 1 of all genes
have zinc finger domains.
31
X-ray Structure of a Zinc Finger Domain
32
Structure of a Three Zinc-Finger Domain of Zif
268 Complexed to an Oligonucleotide Containing
its Recognition Sequence
33
The Specificity of Zinc for Zinc-finger Domains
Kd value 2 pM 5nM 2mM 3mM Metal ion Zn2 Co2
Ni2 Fe3