Total Synthesis of Rapamycin - PowerPoint PPT Presentation
Title:
Total Synthesis of Rapamycin
Description:
Stereochemistry of Eneyne Addition to Aldehyde. Synthesis of Dienylstannane D ... Construction of a C27-C42 Aldehyde. Construction of the C22-C42 Subunit ... – PowerPoint PPT presentation
Number of Views:237
Avg rating:3.0/5.0
Title: Total Synthesis of Rapamycin
1
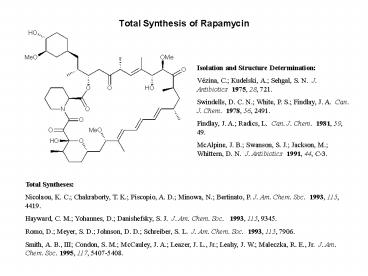
Total Synthesis of Rapamycin
Isolation and Structure Determination Vézina,
C. Kudelski, A. Sehgal, S. N. J. Antibiotics
1975, 28, 721. Swindells, D. C. N. White, P. S.
Findlay, J. A. Can. J. Chem. 1978, 56,
2491. Findlay, J. A. Radics, L. Can. J. Chem.
1981, 59, 49. McAlpine, J. B. Swanson, S. J.
Jackson, M. Whittern, D. N. J. Antibiotics
1991, 44, C-3.
Total Syntheses Nicolaou, K. C. Chakraborty, T.
K. Piscopio, A. D. Minowa, N. Bertinato, P. J.
Am. Chem. Soc. 1993, 115, 4419. Hayward, C. M.
Yohannes, D. Danishefsky, S. J. J. Am. Chem.
Soc. 1993, 115, 9345. Romo, D. Meyer, S. D.
Johnson, D. D. Schreiber, S. L. J. Am. Chem.
Soc. 1993, 115, 7906. Smith, A. B., III Condon,
S. M. McCauley, J. A. Leazer, J. L., Jr.
Leahy, J. W. Maleczka, R. E., Jr. J. Am. Chem.
Soc. 1995, 117, 5407-5408.
2
Immunomodulators
rapamycin
FK-506
cyclosporin A
3
Rapamycins Mechanism of Action
IL-2 Receptor
The Cell Cycle
Restriction Point
?
G1
p70 S6 Kinase
S
G0
Cdc2 Kinase
M
G2
40S Ribosomal Protein S6
Schreiber, S.L. Albers, M. W. Brown, E. J. Acc.
Chem. Res. 1993, 26, 412. Chung, J. Kuo, C. J.
Crabtree, G. R. Blenis, J. Cell 1992, 69, 1227.
4
KCN's Retrosynthetic Analysis of Rapamycin
rapamycin
5
Synthesis of Oxazolidone A
6
Synthesis of Oxazolidone A (continued)
7
KCN's Retrosynthetic Analysis of Rapamycin
rapamycin
8
Synthesis of Subunit B
Z-enolate
9
Synthesis of Subunit B (continued)
10
KCN's Retrosynthetic Analysis of Rapamycin
rapamycin
11
Synthesis of Vinyliodide D
12
Synthesis of Vinyliodide D (continued)
13
KCN's Retrosynthetic Analysis of Rapamycin
rapamycin
14
The Union of A B E
15
Elaboration of EAB
16
The Introduction of D
rapamycin
EABD
17
The End Game Tricarbonyl Formation
Note the first HF step removes the TES groups
and the second HF step removes the TIPS groups
18
The End Game The Stitching Stille Reaction
rapamycin
19
Summary
- Completed the first total synthesis of
(-)-rapamycin. - The longest linear sequence from an article of
commerce consists of thirty-seven steps. - The longest linear sequence from our five
sub-targets is sixteen steps. - Total steps 102
- Instructional applications of the Stille
reaction, oxidation chemistry, chiral
auxiliaries, organosilicons, protective groups,
etc.
20
Smiths Retrosynthetic Analysis ofRapamycin and
Demethoxyrapamycin
21
Synthesis of Iodide A
22
Synthesis of Dithiane B
23
Synthesis of Dithiane C
24
Retrosynthetic Analysis of Rapamycin
25
Synthesis of the Ortho Ester Exploitation of
Alternate Ortho Ester DiastereomerEmployed in
Smiths Latrunculin Synthetic Venture
26
Synthesis of the E and Z Eneynes
27
Mechanism of Olefin Isomerization
28
Stereochemistry of Eneyne Addition to Aldehyde
29
Synthesis of Dienylstannane D
30
Retrosynthetic Analysis of Rapamycin
31
Construction of a C27-C42 Aldehyde
32
Construction of the C22-C42 Subunit
33
Synthesis of DemethoxyrapamycinConstruction of
Advanced ABC Intermediate
34
Retrosynthetic Analysis of Rapamycinand
DemethoxyrapamycinIntroduction of the
Tricarbonyl Segment
35
Tricarbonyl Formation I
O
TBSO
OMe
1) NaH, MeI, 15-crown-5 (80)
O
O
TBSO
O
O
OH
2) HOAc, H
O,THF (86)
2
TMS
3) TBSCl, imid. (97)
TMS
1)
N
E
O
TBSO
OMe
OHC
2) Allylbromide, K2CO3
TMS
DMF (98)
O
O
TBSO
OMe
O
OH
N
TMS
36
Tricarbonyl Formation II
37
Pipecolinyl Acylation
38
Proposed Endgame Bis-Hydrostannylation
39
Attempted Macrocyclizations
40
Preparation of ABC vinylstannane DE vinyl iodide
41
Proposed Endgame Strategy for the Total
Synthesis of Rapamycinand Demethoxyrapamycin
42
Macrocyclization
43
Demethoxyrapamycin
44
Rapamycin
45
Summary
- Developed a highly convergent and efficient total
synthesis of (-)-rapamycin. - The longest linear sequence from an article of
commerce consists of thirty-three steps. - The longest linear sequence from our five
sub-targets is fourteen steps. - After the coupling of the C(1)-C(20) fragment to
the C(22)-C(42) fragment only three steps are
required to complete the synthesis. - Completed the first total synthesis of
demethoxyrapamycin. - The synthesis serves as a structure proof.
- The synthesis establishes our unified synthetic
approach as being amenable to the preparation of
analogs.