Electrolytes - PowerPoint PPT Presentation
1 / 80
Title:
Electrolytes
Description:
acetic acid. solution. potassium dichromate. solution. 4. LOHS AP Chemistry ... Usually molecular compounds like acetic acid (CH3COOH) with ionizable groups (H ... – PowerPoint PPT presentation
Number of Views:360
Avg rating:3.0/5.0
Title: Electrolytes
1
- Electrolytes
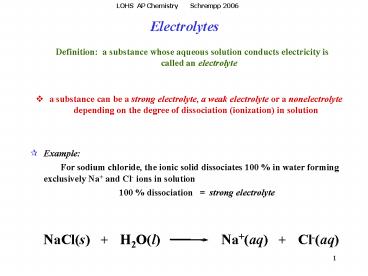
Definition a substance whose aqueous solution
conducts electricity is called an electrolyte
- a substance can be a strong electrolyte, a weak
electrolyte or a nonelectrolyte depending on the
degree of dissociation (ionization) in solution
- Example
- For sodium chloride, the ionic solid
dissociates 100 in water forming exclusively
Na and Cl- ions in solution - 100 dissociation strong electrolyte
2
- AP Chemistry
- Wednesday, Febuary 1
- Turn in free response worksheet 1
- Work on Free Response Equation worksheet 2.
When complete, check your answers on the front
board. Turn in today if finished, or tomorrow
beginning of period. - Video I Came, I Saw, I RedOxd
- Precipitation Demo
- Homework read sections 20.1 and 20.2
- Page 821, do problems 20.2, .4, .6, .8, .10
3
Electrolytes
pure water
acetic acid solution
potassium dichromate solution
4
- Identifying Electrolytes
- Strong electrolytes Substances that dissociate
completely in water. Simple salts like NaCl
that are combination of a metal and a
nonmetal - Weak electrolytes Substances that do not
dissociate fully in water but do form some
ions. Usually molecular compounds like
acetic acid (CH3COOH) with ionizable groups
(H) - Nonelectrolytes Substances that do not
dissociate in water to form ions. Molecular
compounds which are soluble but which
remain intact as the molecule in solution
5
Question What type of electrolytes are these
compounds? a) Epsoms salt MgSO4 . 7
H2O b) Methanol CH3OH c) Acetic acid
CH3COOH
6
Answer a) b) c)
strong electrolyte
nonelectrolyte
weak electrolyte
7
Understanding Predicting Reactions in Solution
- Driving Force a property of the reaction that
can be identified as the reason for product
formation
- Examples
solid formation
8
Types of Reactions
- the reaction type depends on the driving force of
the reaction. There are four basic types
- Formation of an insoluble compound
- Formation of a nonelectrolyte
- Formation of a gas
- Transfer of electrons
9
- Solubility
- certain combinations of cations and anions are
soluble that is they dissolve in water.. - if a compound will not dissolve in water it is
insoluble - if a combination of anion and cation results in
the formation of an insoluble solid, this is a
precipitate
- Example
precipitate
10
Soluble Compounds
Exceptions
Almost all salts of Na, K NH4
Salts of NO3-, ClO3-, ClO4-, CH3CO2-
11
Exceptions
Insoluble Compounds
12
- Net Ionic Equations
- the balanced equation that results from the
omission of all spectator ions is the net ionic
equation - spectator ions are the ions which do not
participate in the reaction
Example Write a balanced net ionic equation for
the reaction of AgNO3 with CaCl2 to produce AgCl
and Ca(NO3)2.
13
Step 1 Write the complete balanced equation
with appropriate stoichiometry
Step 2 Decide on the physical state (eg
solubility) of each compound.
14
Step 3 Recognize that all soluble ionic
compounds dissociate to form ions in
aqueous solution
15
Step 4 Identify the spectator ions and remove
them from the complete ionic equation
to give the net ionic equation. Simplify
the resulting equation in terms of
stoichiometric coefficients.
- The sum of ion charges is the same on
- both sides of the net ionic equation
16
Precipitation Reactions
- Write the net ionic equation for
- the reaction of Pb(NO3)2 with KI.
17
- Acids Bases
- Acid any substance that , when dissolved in
water, increases the concentration of hydrogen
ions, H, in the water - Base any substance that, when dissolved in
water, increases the concentration of hydroxide
ions, OH-, in the water
18
- Strong Vs. Weak
- A strong acid or strong base an acid or base
which ionizes completely in water a strong
electrolyte - A weak acid or base an acid or base which does
not ionize completely in water a weak
electrolyte
19
Acid-Base Reactions I
- Write the net ionic equation for
- the reaction of HNO3 (strong acid) with KOH
(strong base).
Overall Reaction
20
Acid-Base Reactions II
- Write the net ionic equation for
- the reaction of CH3CO2H (weak acid) with
Ca(OH)2 (strong base).
Overall Reaction
2 CH3CO2H(aq) Ca(OH)2(s) Ca(CH3CO2)2(a
q) 2 HOH(l)
21
Some Common Acids Bases
Strong Acids
Strong Bases
HCl, HBr, HI, HNO3, H2SO4, HClO4
NaOH, KOH, Ca(OH)2
Weak Acids
Weak Bases
CH3CO2H, H3PO4, HF, H2CO3
NH3
H2SO4(l) H(aq) HSO4-(aq)
Note
HSO4-(aq) H(aq) SO42-(aq)
22
Gas-Forming Reactions
- The acids of some nonmetal ions are gases and a
small number of aqueous acids easily decompose to
form a gaseous product.
Examples
23
Gas-Forming Reactions
- Write the net ionic equation for
- the reaction of HNO3 with NiCO3.
Overall Reaction
2 HNO3(aq) NiCO3(s)
Ni(NO3)2(aq) H2CO3(aq)
24
- Properties of Compounds in Aqueous Solution
- Aqueous solution a solution of any substance or
substances dissolved in water
- Example
- Solid sodium chloride dissolves in water to
give an aqueous solution of sodium cations and
chloride anions
aqueous solution of sodium chloride
25
- Oxides of Metals Nonmetals
- If a nonmetal oxide is dissolved in water an
acidic solution results. This compounds is known
as an acidic oxide - If a metal oxide is dissolved in water a basic
solution results. This compounds is known as a
basic oxide
26
Summary Types of Reactions
- the reaction type depends on the driving force of
the reaction. There are four basic types
Reaction Type Driving
Force Precipitation Reaction Formation of an
insoluble compound Acid-Base Neutralization Forma
tion of a nonelectrolyte (water) Gas-Forming Ev
olution of a water insoluble gas Oxidation
-reduction Transfer of electrons
27
Chemistry-140 Lecture 11
Types of Reactions
- the reaction type depends on the driving force of
the reaction. There are four basic types
Reaction Type Driving
Force Precipitation Reaction Formation of an
insoluble compound Acid-Base Neutralization Forma
tion of a nonelectrolyte (water) Gas-Forming Ev
olution of a water insoluble gas Oxidation
-reduction Transfer of electrons
28
Oxidation-Reduction Reactions
- Write the net ionic equation for
- the reaction of Cu with AgNO3.
29
Oxidation-Reduction Reactions
30
Redox Reactions and Electron Transfer (oil rig,
leo goes ger)
- All oxidation-reduction reactions involve the
transfer of electrons between substances
31
Oxidation Numbers
Question How can you tell an
oxidation-reduction reaction when you see one ?
Answer Look for a change in oxidation number
for an element(s)
Example
silver is reduced from oxidation state 1 to
oxidation state 0
32
Guidelines For Determining Oxidation Numbers
33
Guidelines For Determining Oxidation Numbers
34
Guidelines For Determining Oxidation Numbers
- The oxidation number of H is 1 and of O is -2 in
most compounds.
- Exceptions are very few BUT. binary compounds
between metals and hydrogen are hydrides (H-) and
H has oxidation state -1. - Exceptions are very few BUT peroxide O22- has
oxygen in oxidation state -1.
- The algebraic sum of the oxidation numbers in
neutral compounds must be 0 in a polyatomic ion,
the sum must be equal to the ion charge.
35
Assigning Oxidation Numbers
Question What are the oxidation numbers
of a) Lithium and oxygen in
Li2O b) Manganese and oxygen in MnO4-
36
Common Oxidizing Agents (species are reduced, i.e
they gain electrons)
37
Common Reducing Agents (these species are
oxidized, i.e., they loose electrons)
38
Products of Simple Redox Reactions
- Write the net ionic equation for the
- reaction of Na with Cl2
- Na is a good reducing agent
- Cl2 is a good oxidizing agent
- the overall redox equation would then be
39
Products of Simple Redox Reactions
- Write the net ionic equation for the
- reaction of K with H2O
- K is a good reducing agent
- H can be reduced to molecular hydrogen
- the overall redox equation would then be
40
Products of Simple Redox Reactions
- Write the net ionic equation for the
- reaction of Fe2O3 with Al
- Al is a good reducing agent
- a metal oxide (Fe2O3) can be reduced back to the
metal
- the overall redox equation would then be
41
Summary Types of Reactions
- the reaction type depends on the driving force of
the reaction. There are four basic types
Reaction Type Driving
Force Precipitation Reaction Formation of an
insoluble compound Acid-Base Neutralization Forma
tion of a nonelectrolyte (water) Gas-Forming Ev
olution of a water insoluble gas Oxidation
-reduction Transfer of electrons
42
Some Problems
Question Write the net ionic equation for the
reaction of aqueous solutions of K2CO3 and HClO4.
43
Some Problems
Answer Write a balanced equation with
appropriate physical states indicated.
and Remember
44
Some Problems
Express the equation in a fully ionic form.
Eliminate spectator ions and reduce to simplest
stoichiometry.
45
Some More Problems
Question Write the net ionic equation for the
reaction of Ca with aqueous HCl.
46
Some More Problems
Answer Write a balanced equation with
appropriate physical states indicated.
because
- H can be reduced to molecular hydrogen
47
Some More Problems
Express the equation in a fully ionic form.
Eliminate spectator ions and reduce to simplest
stoichiometry.
48
Solubility Rules
- Alkali metals and NH4 compounds are soluble.
- Nitrates(NO3?), acetates (CH3CO2?), chlorates
(ClO3?), and perchlorates(ClO4?) are soluble. - Chlorides(Cl?), bromides(Br?), iodides(I?), are
soluble except for Silver(Ag),mercury(I)(Hg22),
and lead(II)( Pb2) halides. - Sulfates(SO4?2) are soluble except for Sr2,
Ba2, Pb2, and Hg22. - Hydroxides(OH?) are insoluble except for alkali
metals and NH4 (see1). - Sulfides(S?2), carbonates(CO3?2),
phosphates(PO4?3), and chromates(CrO4?2) are
insoluble except for alkali metals and NH4
(see1).
49
Classifying Reactions by Type of Chemistry
50
Classifying Reactions by Type of Chemistry
- Precipitation AX BZ ?? AZ BX
- Acid Base HX BOH ?? BX H2O
- Gas Evolution
- H2X BCO3 ? H2O CO2(g) BX
- H2X BSO3 ? H2O SO2(g) BX
- NH4X BOH ? H2O NH3(g) BX
- Oxidation Reduction A2 B ?? A B2
- Combustion CxHxOxO2?CO2 H2O
51
Classifying Reactions by what Atoms Do
52
Classifying Reactions by what Atoms Do
- Combination/Synthesis A Z ?? AZ
- Decomposition AZ ?? A Z
- Single Displacement A BZ ?? AZ B
- Double displacement AX BZ ?? AZ BX
- Neutralization HX BOH ?? BX H2O
53
Predicting ReactionsDouble Displacement
54
BaCl2 ZnSO4 ?
- Conventional equation
- BaCl2(aq) ZnSO4(aq) ? BaSO4(s) ZnCl2(aq)
- Total ionic equation
- Ba2(aq) 2Cl-1(aq) Zn2(aq) SO4-2(aq) ?
BaSO4(s) Zn2 (aq) 2Cl-1(aq) - Net ionic equation
- Ba2(aq) SO4-2(aq) ? BaSO4(s)
55
AgNO3 Na2SO4?
- AgNO3 Na2SO4? NR
56
(NH4)2CO3 CaCl2 ?
- (NH4)2CO3 CaCl2 ? 2 NH4Cl CaCO3(s)
57
Na2S ZnCl2 ?
- Na2S ZnCl2 ?2 NaCl ZnS(s)
58
K3PO4 Sr(NO3)2 ?
- 2 K3PO4 3 Sr(NO3)2 ? 6 KNO3 Sr3(PO4)2 (s)
59
Mg(NO3)2 NaOH ?
- Mg(NO3)2 2 NaOH ? Mg(OH)2(s) 2 NaNO3
60
Ba(OH)2 H3PO4 ?
- 3 Ba(OH)2 2 H3PO4 ? Ba3(PO4)2(s) 6 H2O
61
HClO4 NaOH ?
- HClO4 NaOH ? H2O(l) NaClO4
62
H3PO3 NH3 ?
- H3PO3 3 NH3 ? (NH4)3PO3
63
CH3COOH KOH ?
- CH3COOH KOH ? CH3COO-K H2O
64
NH4Cl KOH ?
- NH4Cl KOH ? (NH4OH KCl) ?
- NH3 HOH KCl
65
K2CO3 HCl ?
- K2CO3 2 HCl ? (H2CO3 2 KCl)
- ?H2O CO2 2 KCl
66
K2SO3 HCl ?
- K2SO3 2 HCl ? (H2SO3 2 KCl)
- ?H2O SO2 2 KCl
67
H2CO3 Ca(NO3)2 ?
- H2CO3 Ca(NO3)2 ? CaCO3(s) 2 HNO3
68
Fe(NO3)3 NH3 ?
- Fe(NO3)3 3NH3 3H2O? Fe(OH)3(s) 3NH4NO3
69
More
- K2S CuSO4 ?
- Na2CrO4 Pb(C2H3O2)2 ?
- ZnBr2 K3PO4 ?
- KOH NH4Cl ?
- NH3 HCN ?
70
Predicting Reactions Single Displacement (AKA
Single Replacement)
71
(No Transcript)
72
Pb Zn(NO3)2 ?
73
Fe HCl ?
74
Cu AgNO3?
75
Cr Zn(NO3)2 ?
76
Pb Sn(NO3)2 ?
77
H2 NiCl2 ?
78
Cr NiCl2 ?
79
H2 Au(NO3)2 ?
80
- Classifying/Predicting Reactions
- Five types of reactions are possible in the AP
Exam - Double Replacement (metathesis) Reactions
- Two binary ionic compounds (including Acids)
- Product is a gas, a precipitate, or a weak
electrolyte