How do metals deform plastically - PowerPoint PPT Presentation
1 / 18
Title:
How do metals deform plastically
Description:
(b) To minimize this compressive strain, impurity atoms tend to localize adjacent ... Highly strained lattice (many atoms out of place) ... – PowerPoint PPT presentation
Number of Views:177
Avg rating:3.0/5.0
Title: How do metals deform plastically
1
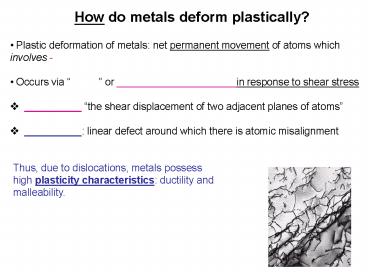
How do metals deform plastically?
- Plastic deformation of metals net permanent
movement of atoms which involves - - Occurs via or _____________________i
n response to shear stress - __________ the shear displacement of two
adjacent planes of atoms - __________ linear defect around which there is
atomic misalignment
Thus, due to dislocations, metals possess high
plasticity characteristics ductility and
malleability.
2
Dislocations and Plastic Deformation
- For most crystalline materials, plastic
deformation occurs by slip where one plane of
atoms slides over adjacent plane by dislocation
motion
?
- (a) Apply ? ? bonds break in B plane ?
- (b) bonds form in A plane Edge dislocation has
moved 1 atomic distance - (c) Edge dislocation continues to move in
increments until extra half plane of atoms exits
? forms step at surface
Slip Plane crystallographic plane along which
dislocation moves
3
Dislocation Motion
Edge dislocation dislocation line (?) moves
parallel to applied stress (?)
slip step
Screw dislocation dislocation line (C) moves
perpendicular to applied stress (?)
slip step
For both
Adapted from Fig. 7.2, Callister 7e.
4
Features of Dislocations
- 1. Dislocations are introduced/formed via
- 2. Dislocation Density
- total of dislocations/unit volume of
material or of dislocations which
intersect a unit area of a random surface
section - 103 per mm2 (slowly solidified) 1010 per
mm2 (highly deformed) - dislocation density increases with
________________________ - Ceramics 102 104 per mm2 (have less
plasticity vs. metals)
5
Single Crystal Slip
Single Zinc Crystal
before deformation
after tensile elongation
slip steps
Adapted from Fig. 7.9, Callister 7e.
6
Mechanism to Strengthen Metals
Reduce dislocation motion (slip)
____________________________
Metal becomes stronger ??y ?TS (?
ductility) Metal also becomes harder resists
local plastic deformation
7
3 Strategies for Strengthening Metals
1. Reduce Grain Size
- Grain Boundaries (see next slide for
definition) - disrupts/hinders motion of dislocations through
a material improve strength - - grain boundaries act as _______________ to
dislocation motion - a dislocation passing into grain B from grain
A will have to change its
direction of motion - High angle grain boundary increase strength
(vs. low angle) - Fine grained (having small grains) increase
strength (vs. coarse-grained)
Why?
8
- Recall what a grain boundary is
- Separates two small grains or crystals having
different crystallographic orientations in
polycrystalline materials - the interface separating two adjoining grains
having two crystallographic orientations
- Features
- Different degrees of atom misalignment
- Atoms bonded less regularly along grain boundary
- Higher energy
- Higher chemical reactivity
- Impurity atoms tend to segregate along grain
boundaries
9
3 Strategies for Strengthening Metals
2. Solid-Solution Strengthening
- Alloys are _________________ than pure metals
- Alloying increases ?y and TS but decreases EL
10
Ex Solid-Solution Strengthening in Copper
Tensile strength yield strength
increase with wt Ni
Ex Solid-Solution Strengthening in Titanium
11
How does solid solution strengthening work?
- Driving force of solid solution strengthening is
_______________________ - _____________________________
- Dislocations impurity atoms introduce lattice
distortion ? - Impose lattice strains to surrounding atoms ?
- Impurity atoms move to locations to reduce
lattice strain ? - Impurity atoms segregate around dislocations to
reduce lattice strain ? - If dislocation were to move, impurity atoms
would be moved from dislocation and the low
stain state would be destroyed ? - Thus, movement of dislocation inhibited
increase strength by decreasing plastic
deformation.
12
Case 1 Impurity atoms smaller than host atoms
- For edge dislocation ___________ dislocation
line, the atoms are crowed ? creates
___________________ strain - (a) An impurity atom that is smaller than a host
atom for which it substitutes - Impurity atom nearest neighbors (?) move in
closer ? pull away from other nearest neighbors ?
creates tensile strain in surrounding crystal
lattice. - (b) To minimize this tensile strain and the
compressive strain above the dislocation line,
impurity atoms tend to localize adjacent to the
dislocation line and above the slip plane (at
high density side).
13
Compressive strain above slip plane
Tensile strain around impurity atoms
Impurity atoms segregate at dislocation above the
slip plane (on high density side) - Reduces
crowding/compressive strain above slip plane -
Tensile strain cancels out some of the
compressive strain
14
Case 2 Impurity atoms larger than host atoms
For edge dislocation above dislocation line, the
atoms are crowed ? creates _________________
strain. (a) An impurity atom that is larger
than a host atom for which it substitutes Impurit
y atom pushes against nearest neighbors (?) ?
push against other nearest neighbors ? creates
compressive strain in surrounding crystal
lattice. (b) To minimize this compressive
strain, impurity atoms tend to localize adjacent
to the dislocation line and below the slip plane
(low density side).
15
Compressive strain above slip plane
Above slip plane, the atoms are more spread
out can accommodate larger impurity atoms If
impurity atoms were above slip plane, compressive
strain would be increased.
16
3 Strategies for Strengthening Metals
3. Strain Hardening / Work Hardening /
Cold-Working
- Increase strength hardness of a ductile metal
by plastically deforming below its
recrystallization temperature - Recrystallization Temp min. temp. at which
complete recrystallization of an alloy will occur
in 1 hr
Metals are often processed at low temperatures
such that their cross-sectional area (Ad) is
reduced as they are plastically deformed
Adapted from Fig. 11.8, Callister 7e.
17
Express degree of plastic deformation as
cold-worked (rather than strain)
Increase CW ?
18
How does cold working work?
Cold working ? _________________________________
________ ? ______________________________________
_____ ? Highly strained lattice (many atoms out
of place) ? Dislocation motion
____________________________________________
motion would increase lattice strain