Physician Assessment of Peripheral Neuropathy NCI CTC Grade - PowerPoint PPT Presentation
1 / 9
Title:
Physician Assessment of Peripheral Neuropathy NCI CTC Grade
Description:
... of Peripheral Neuropathy. NCI CTC Grade Cumulative Dose ... Patient Assessment of Peripheral Neuropathy. Total Score by Cumulative Dose. Source: NDA Addendum. ... – PowerPoint PPT presentation
Number of Views:49
Avg rating:3.0/5.0
Title: Physician Assessment of Peripheral Neuropathy NCI CTC Grade
1
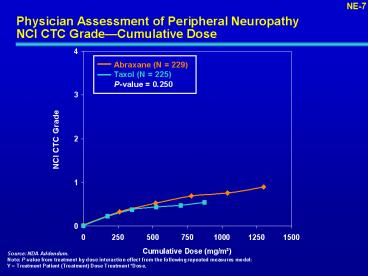
Physician Assessment of Peripheral NeuropathyNCI
CTC GradeCumulative Dose
NE-7
Abraxane (N 229) Taxol (N 225) P-value 0.250
NCI CTC Grade
Source NDA Addendum. Note P-value from
treatment by dose interaction effect from the
following repeated measures model Y Treatment
Patient (Treatment) Dose Treatment Dose.
2
Patient Assessment of Peripheral NeuropathyTotal
Score by Cumulative Dose
NE-8
Abraxane (N 229) Taxol (N 225) P-value 0.202
Total Score
Source NDA Addendum. Note P-value from
treatment by dose interaction effect from the
following repeated measures model Y Treatment
Patient (Treatment) Dose Treatment Dose.
3
Patients with Grade 3 Sensory Neuropathy Time to
Improvement to Grade 1 or 2 (Based on AE Data)
NE-9
Abraxane (N 24) Taxol (N 5) Censored P-value
0.028
Proportion Not Improved
Source NDA Addendum. Note P-value from log-rank
test.
4
Examples of Drugs Approved as 505(b)(2) NDAs
RG-2
RLD 505(b)(2) Product
Taxol (paclitaxel) Injection (BMS) Abraxane for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) (Abraxis BioScience, Inc.) Change to an approved drug (different excipient)
Genotropin (somatropin for injection) (Pfizer) Omnitrope (somatropin rRNA origin) for injection (Sandoz, Inc.) Change to an approved drug (follow-on protein/recombinant source)
Premarin (conjugated estrogen tablets, USP) (Wyeth) Cenestin tablets (estrogens, conjugated synthetic A) (Duramed) Change to an approved drug (immediate release tablet/synthetic estrogens)
5
CA008 Summary of Paclitaxel PharmacokineticParam
eters for Abraxane and Taxol
PK-3
Source Abraxane NDA
6
Biodistribution of Abraxane andTaxol Are Similar
PK-11
7
Time to Progression Analysis for Phase 3 Study
TI-1
- The following two analyses of TTP have been
conducted - TTP based on all on-therapy Investigator response
assessments. - post-approval commitment, June 2005
- TTP based on the Independent Radiology Laboratory
(IRL) response assessments and Investigator
response assessments - labeling supplement, July 2006
- It makes use of all available response
assessments including the IRL assessments for
cycles 16 and all Investigator assessments, and - It uses a conservative approach through cycles
16 by selecting the earliest date of progression
between the IRL assessments and Investigator
assessments - These TTP data are currently under review with
the FDA
8
Abraxane Prolonged Time to Tumor
ProgressionCA012 Secondary Endpoint
TI-2
Time to Tumor Progression
Time to Tumor Progression
Abraxane (N 233) Taxol (N 227) P-value
0.002 Hazard Ratio 0.721
Proportion Not Progressed
Investigator assessmentJune 2005(NDA Post
marketing commitment)
Investigator assessmentIndependent radiology
reviewJuly 2006(Labeling supplement)
Note P-value from log-rank test.
9
Time to Disease ProgressionBlinded Independent
Radiology Laboratory Assessment
TI-3
Proportion not Progressed
Abraxane (N 215) Taxol (N 214) P-value
0.003 HR 0.519
Source data on file Abraxis BioScience Note
P-value from log-rank test.