Bioenergetics - PowerPoint PPT Presentation
1 / 28
Title:
Bioenergetics
Description:
Do accelerate rate of reaction (kinetics) Highly specific for substrate/reactant ... No relationship between G and rate of a reaction (kinetics) ... – PowerPoint PPT presentation
Number of Views:41
Avg rating:3.0/5.0
Title: Bioenergetics
1
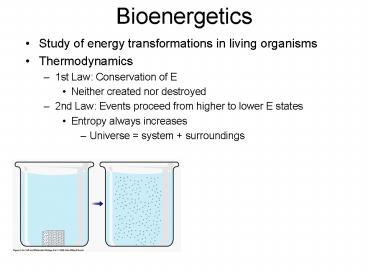
Bioenergetics
- Study of energy transformations in living
organisms - Thermodynamics
- 1st Law Conservation of E
- Neither created nor destroyed
- 2nd Law Events proceed from higher to lower E
states - Entropy always increases
- Universe system surroundings
2
Bioenergetics
- (E content of system) ?H (useful free E) ?G
(E lost to disorder) T?S - Gibbs Free Energy ?G ?H - T?S
- If ?G negative, then rxn is exergonic,
spontaneous - If ?G positive, then rxn is endergonic, not
spontaneous - Standard conditions (?G) 25oC, 1M each
component, pH 7, H2O at 55.6M
3
Bioenergetics
- A B lt--gt C D
- Rate of reaction is directly proportional to
concentration of reactants - At equilibrium, forward reaction backward
reaction - k1AB k2CD
- Rearrange
- k1/k2 (CD)/(AB) Keq
- Relationship between ?G and Keq is
- ?G -2.303 R T log Keq
- If Keq gt1, ?G is negative, rxn will go forward
- If Keq lt1, ?G is positive, rxn will go backward
4
(No Transcript)
5
- ?G is a fixed value at standard conditions
- ?G under actual cellular conditions can be
different - e.g., for ATP hydrolysis inside a cell, can
approach ?G -12 kcal/mol - We will work with ?G values
6
Coupling endergonic and exergonic rxns
Glutamic acid (Glu) NH3 --gt Glutamine
(Gln) ?G3.4 kcal/mol
ATP --gt ADP Pi ?G-7.3
kcal/mol -----------------------------------------
-----------------------------------------------
Glu ATP NH3 --gt Gln ADP
Pi ?G-3.9 kcal/mol Glutamyl phosphate is the
common intermediate
7
ATP --gt ADP Pi ?G -7.3 kcal/mol ADP Pi
--gt ATP ?G 7.3 kcal/mol C(diamond) O2
--gt CO2 ?G -94.8 kcal/mol PEP --gt pyruvate
Pi ?G -14.8 kcal/mol C(graphite) O2 --gt
CO2 ?G -94.1 kcal/mol P-creatine --gt
creatine Pi ?G -11.0 kcal/mol G6-P --gt
glucose Pi ?G -3.0 kcal/mol 1,3-BPG --gt
3PG Pi ?G -12.5 kcal/mol ------------------
--------------------------------------------------
-------------------- What is ?G of PEP ADP
--gt pyruvate ATP ------------------------------
--------------------------------------------------
-------- What is ?G of G6-P ADP --gt glucose
ATP What is ?G of P-creatine ADP --gt
creatine ATP What is ?G of C(s, diamond) --gt
C(s, graphite)
8
Equilibrium vs steady state
- Cells are open systems, not closed systems
- O2 enters, CO2 leaves
- Allows maintenance of reactions at conditions far
from equilibrium
O2
9
- Reqd in small amounts
- Not altered/consumed in rxn
- No effect on thermodynamics of rxn
- Do not supply E
- Do not determine product/reactant ratio (Keq)
- Do accelerate rate of reaction (kinetics)
- Highly specific for substrate/reactant
- Very few side reactions (i.e. very clean)
- Subject to regulation
- No relationship between ?G and rate of a reaction
(kinetics) - Why might a favorable rxn not occur rapidly?
Biological Catalysts
10
(No Transcript)
11
Overcoming the activation energy barrier (EA)
- Bunsen burner CH4 2O2 --gt CO2 2H2O
- The spark adds enough E to exceed EA (not a
catalyst) - Metabolism burning glucose
- Enzyme lowers EA so that ambient fluctuations in
E are sufficient
12
Overcoming the activation energy barrier (EA)
- Bunsen burner CH4 2O2 --gt CO2 2H2O
- The spark adds enough E to exceed EA
- Metabolism burning glucose
- Enzyme lowers EA so that ambient fluctuations in
E are sufficient
Catalyst shifts EA line to left lt---
13
How to lower EA
- The curve peak is the transition state (TS)
- Enzymes bind more tightly to TS than to either
reactants or products
14
How to lower EA
- Mechanism form an Enzyme-Substrate (ES) complex
at active site
15
How to lower EA
- Mechanism form an Enzyme-Substrate (ES) complex
at active site - Orient substrates properly for reaction to occur
- Increase local concentration
- Decrease potential for unwanted side reactions
16
How to lower EA
- Mechanism form an Enzyme-Substrate (ES) complex
at active site - Enhance substrate reactivity
- Enhance polarity of bonds via interaction with
amino acid functional groups - Possibly form covalent bonded intermediates with
amino acid side chains
17
How to lower EA
- Possibly form covalent bonded intermediates with
amino acid side chains - Serine protease mechanism
18
How to lower EA
- Possibly form covalent bonded intermediates with
amino acid side chains - Serine protease mechanism
19
How to lower EA
- Mechanism form an Enzyme-Substrate (ES) complex
at active site - Induce bond strain
- Alter bonding angles within substrate upon
binding - Alter positions of atoms in enzyme too Induced
fit
20
Induced fit
21
Induced fit
22
Enzyme kinetics The Michaelis-Menten Equation
S lt--gt P At low S, rate/velocity is slow,
idle time on the enzyme At very high S,
rate/velocity is maximum (Vmax), enzyme is
saturated V Vmax S/(S Km) Km S at
Vmax/2 A low Km indicates high enzyme affinity
for S (0.1mM is typical)
23
Enzyme kinetics pH and temperature dependence
24
Enzyme inhibitors
- Irreversible
- Form a covalent bond to an amino acid side chain
of the enzyme active site - Blocks any further participation of the enzyme in
catalysis
25
Enzyme inhibitors
- Reversible
- Competitive
- bind at active site
- Steric block to substrate binding
- Km increased
- Vmax not affected (increase S can overcome)
26
Enzyme inhibitors
- Reversible
- Noncompetitive
- Bind at discrete site, not overlapping active
site - Subtly alter enzyme structure reducing catalysis
- Km not affected
- Vmax decreased, (increase S cannot overcome)
Noncompetitive
Competitive
27
(No Transcript)
28
Drug discovery
- Average cost to market 1B
- Average time to market 13 years
- Size of market 170B per year in US
- S. aureus infections are a problem in hospital
settings - Drug targets
- Metabolic rxns specific to bacteria
- Sulfa drugs (folic acid biosynthesis)
- Cell wall synthesis
- Penicillin, methicillin, vancomycin
- DNA replication, transcription, translation
- Ciprofloxacin (DNA gyrase)
- Tetracyclins (ribosome)
- Zyvox (ribosome)
- Introduced in 2000, resistance observed within 1
year of use