The need of deuteration - PowerPoint PPT Presentation
1 / 28
Title:
The need of deuteration
Description:
The need of deuteration. Why is necessary to enrich the protein with 2H? Deuteriation reduces the relaxation rates of NMR-active ... of aliphatic amino acids ... – PowerPoint PPT presentation
Number of Views:375
Avg rating:3.0/5.0
Title: The need of deuteration
1
The need of deuteration
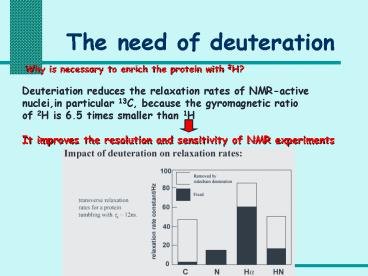
Why is necessary to enrich the protein with 2H?
Deuteriation reduces the relaxation rates of
NMR-active nuclei,in particular 13C, because the
gyromagnetic ratio of 2H is 6.5 times smaller
than 1H It improves the resolution and
sensitivity of NMR experiments
2
Which is the ideal level of deuteration?
It depends from the size of the protein In
general ? for ?c ? up to 12 ns (20 Kda) 13C/15N
labeling ? for ?c ? up to 18 ns (35 Kda) 13C/15N
labeling and fractional deuteration ? for ?c
above 18 ns 13C/15N labeling selective
protonation and background deuteration It
depends from the type of NMR experiments
3
The problem to express a deuterated protein
Incorporation of 2H reduces growth rate of
organisms (up to 50) and decreases protein
production as a consequence of the isotopic
effect.
Deuterium labeling requires conditions different
with respect to 13C and 15N enrichment and could
require bacteria adaptation
4
Fractional deuteration
Random fractional deuteration can be obtained up
to a level of 70-75 , in a media with 85 D2O
with protonated glucose, without bacteria
adaptation
Expressing culture labeled gt20 h
Preinduction culture labeled 2-6 hours
OD6000.3-1.2
O/N culture unlabeled
As for 13C-15N labelling all the conditions
(strain, glucose conc. time of induction, etc.)
must be optimized for each protein!!
5
An example expression of 13C-1N-2H HSOD
Human SOD (MW 32000) was deuterated up to 70, by
growing E.coli in minimal medium containing 80
D2O Conditions were the following O/N culture in
LB was used to inoculated 1 l MM 80
D2O Induction was performed after 6 hours (OD600
0.5). Cells were harvested 8 hours after
induction. From 1 l culture we obtained 15 mg of
pure protein
MM composition (1 l) 15N M9 10 x 100 ml 13C
glucose 4 50 ml Mg/Ca/VitB1/Goodies 100x
10 ml Amp 4 5 ml D20 800 ml H2O
35 ml O/N inoculum 10 ml
Viezzoli et al. Eur.J.Biochem. 2002, 269,
1905-1915
6
Deuterium incorporation
Fractional deuteration of recombinant proteins
determined using mass spectroscopy. ( )
deuteration with 2H2O only. ( ) deuteration
with 2H2O and perdeuterated glucose.
OConnell et al. Anal.Biochem. 1998, 265, 351-355
7
Perdeuteration
? Perdeuteration can require a gradual adaptation
of bacteria to increasing concentration of D2O.
? Bacterial strains must be accurately selected
in order to choose that which better acclimates
to D2O media. ? For each strain one or more
colony must be selected which better survives in
high level of D2O concetration
8
A protocol for bacteria adaptation to deuterated
medium
40 D2O
60 D2O
80 D2O
99 D2O
O/N Inoculum in unlabelled medium
Massive culture 99 D2O
Gkycerol stock 40 D2O
Glycerol stock 60 D2O
Glycerol stock 80 D2O
Glycerol stock 99 D2O
9
Is it possible to avoid the adaptation phase?
Wüthrich lab has recently experimented a culture
minimal medium supplemented with deuterated
algal hydrolysate which allows us to eliminate
cells pre-conditioning.
Composition of the Celtone-supplemented
media Basic minimal medium 800 ml H2O or
D2O 100 ml M9 solution 2 ml 1M MgSO4 1
g NHCl 1 g D-glucose Vitamin mix and
trace elements 10 ml of Vitamin mix 2 ml
Trace elements solution Aminoacids supplements
1-3 g deuterate algal lysate (CELTONE)
dissolved at 30 ml/ml antibiotics
Wüthrich K. et al J.Biomol.NMR 2004,29,
10
Is it possible to avoid the adaptation phase?
SOME RESULTS
Medium composition Deuteration Advantage/disadvant
ages Minimal medium on 60-92 no N-H/N-D
exchange problems Glucose-d Celtone-d intermed
iate deuteration can be achieved in H2O Minimal
medium on 95-97 high deuteration Glucose-d
Celtone-d in D2O
Wüthrich K. et al J.Biomol.NMR 2004,29,
11
Specific labeling
Labeling of a protein can be easily achieved on
specific residues with 2 strategies
?In a mineral medium, containing glucose and
complemented with the labelled aminoacids. A
mixture of the other unlabeled aminoacids can be
added to prevent any conversion of the
labeled aminoacids
?In a complete labelled medium, containing great
amount of all unlabeled aminoacids except those
which are expected to be labeled
12
Specific labeling the main problem
The most important problem encountered is the
metabolic conversions of the labeled aminoacids
which might occur during anabolism and/or
catabolism.
How to prevent this?
?Use an auxotrophic strain.
? Use a prototrophic strain with high
concentration of aminoacids to inhibit some
metabolic pathways. An example Labeling of a
protein with 13C15N Lys can be performed in
unlabeled media with high level of 13C15N Lys,
12C14N Thr, and 12C14N Met to prevent lysine
biosinthesis from aspartate conversion.
13
Expression of human SOD selectively deuterated on
His residues
? A prototrophic E. coli strain trasformed with
a vector harbouring SOD gene was grown in the
following medium 100 ml M9 salts 10 x 50 ml
M9 mix 20 x 100 ml AA mix w.o. His 100
?l vit B1 (1) 1 ml Trace metals 11 ml
deuterated His (5) 10 ml ampicillin (2) H2O
to 1 l
Culture was grown following the regular protocol
for HSOD expression
14
Medium composition
Expression of human SOD selectively deuterated on
His residues.
M9 salts (10X) 1l KH2PO4 30 g K2HPO4 70 g NaCl
5 g NH4Cl 10 g
M9 mix (20X) 1l glucose 200 g MgSO4.7H2O 4.9
g CaCl2 .2H2O 0.28 g
AA mixture (10 X) Ala 272 mg Lys 256 mg Arg
366 mg Phe 128 mg Asp 304 mg Ser 152 mg Cys
24 mg Thr 152 mg Glu 576 mg Trp 100 mg Gly 128
mg Tyr 64 mg Ile 160 mg Val 184 mg Leu 232
mg Met 160 mg
15
A successfull example from our laboratory
Viezzoli MS et al. Inorg. Chem. 1990, 2917,
1438-1440
16
Specific labeling for assignment of 13C and 1H
methyl from Ile, Leu, Val
? Full deuteration precludes the use of NOEs for
structure determination.
How to overcome the problem?
Reintroduction of protons by using labeled
amino acids
Reintroduction of protons by using methyl
selectivelly protonated metabolic precursors of
aliphatic amino acids
17
Expression of malate synthase G with selectively
protonated Ile, Leu, Val methyls
A methyl protonated sample of MSG was obtained
from a culture of E.coli BL21(DE3)pLys cells
traformed with the plasmid pMSG-B encoding MSG.
The protein was expressed in 2 l of D2O
medium using 2g/l of 13C2H-glucose and 1g/l of
15NH4Cl. 1 hour before induction 120 mg of
2-keto-3,3-d2-1,2,3,4-13C-butyrate and 200 mg
of 2-keto-3-methyl-d3-3-d1-1,2,3,4-13C-butyrate
The growth was continued for 7h at 37 C.
18
1H-13C CT-HMQC spectrum of a sample of MSG with
methyl protonated Ile, Leu and Val
Tugarinov et al. J.Am.Chem.Soc. 2003, 125,
13868-13878
19
Specific protonation at ring carbons of Phe, Tyr,
and Trp on deuterated proteins
? NOEs involving aromatic protons are an
important source of distance restraints in the
structure calculation of perdeuterated proteins.
A selective reverse labeling of Phe, Tyr and Trp
has been performed in perdeuterated proteins,
using shikimic acid, a precursor of the aromatic
rings. In this way the aromatic rings of the
aminoacids are partially protonated (50)
Rajesh S. et al. J.Biomol.NMR 2003, 27, 81-86
20
Specific protonation at ring carbons of Phe, Tyr,
and Trp on deuterated proteins
21
Specific protonation at ring carbons of Phe, Tyr,
and Trp on deuterated proteins
The aromatic rings of the aminoacids are
partially protonated (40-56). Higher level of
protonation are observed in E.coli strains
overexpressing a membrane bound transporter of
shikimate
Complete protonation can be achieved using an
auxotrophic strain defective in shikimate
production
22
Expression of isotopicaly labeled proteins in
other bacteria
An example ? Expression of gramicidin in B.
brevis Two different strain have been choosen and
grown on different labelled media.
Base medium 11.3 g NaH2PO4 3.5 g NaH2PO4 0.5 g
NaCl 10 g glucose 0.5 mmol MgSO4 1.5 ?mol MnSO4 2
?mol FeSO4 0.2 ?mol CuSO4 0.75 ?mol FeCl3 0.1
?mol KI 0.1 mmol CaCl2 10 nmol thiamin 1 nmol
folic acid 1 nmol boric acid
The base medium is supplemented
with Peptone Peptone yeast extract DL-leucine Le
ucine pipecolic acid All aminoacids NH4Cl Pipeco
lic acid
Bas Vogt TC et al. J. Biomol. NMR 2003, 26, 1-11
23
Expression of isotopically labeled proteins in B.
brevis
24
Expression of isotopically labeled proteins B.
brevis comparison between several media
25
Expression of a deuterated protein in P. Pastoris
? Expression of MSP1-19, produced by P.
falciparum, in P. Pastoris (strain SMD1168) as a
partially deuterated and 13C/15N labeled protein
Buffered minimal media were supplied with glucose
(BMGlc) or methanol (BMMe) as carbon source. The
isotope labeled compound were D2O (NH4)2SO4 D4-me
thanol 99.8 13C methanol 99.3 13C glucose 99
13C-d4-methanol, 13C 99, D 99
Morgan WD et al. J et al. J. Biomol. NMR 2000,
17, 337-347
26
Expression of a deuterated protein in P. Pastoris
?Growth characteristics compared between cells
non adapted and gradually adapted (from 25 to
95) to growth in deuterated media, in
pre-induction (glucose) media and inducing
(methanol) media
27
Expression of a deuterated protein in P. Pastoris
?Comparison expression in adapted and non-adapted
cells, in 95 D2O
Average yield from non adapted cells were 66
of the yield from adapted cells. Adaptation of
the cells to growth at High D2O concentration
was found to improve cell growth rate but was not
essential for expression.
28
Expression of a deuterated protein in P. Pastoris
?Residual protonation in deuterated MSP1 samples
2D-1H13C-HSQC spectra of 13C2H labeled MSP1
protein
Non deuterated control
95 D2O/CH3OH.
Deuteration level is 85 if culture is grown in
95 D2O and CH3OH. If CD3OD is used instead
of CH3OH 90-95 deuteration was achieved with
samples grown in 95 D2O/ CD3OD
95 D2O/CD3OD.