Metabolism - PowerPoint PPT Presentation
1 / 54
Title:
Metabolism
Description:
Induced fit brings reactive side chains together from the active site into ... Allosteric effects regulate metabolism. End-product or feedback inhibition ... – PowerPoint PPT presentation
Number of Views:39
Avg rating:3.0/5.0
Title: Metabolism
1
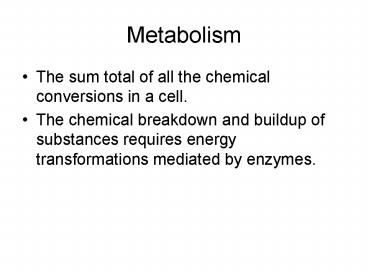
Metabolism
- The sum total of all the chemical conversions in
a cell. - The chemical breakdown and buildup of substances
requires energy transformations mediated by
enzymes.
2
Energy
- Definitions of energy
- Capacity to do work (physicists definition)
- Capacity for change (biochemists definition)
- All living things obtain energy from the
environment. - Energy can be transformed from one type to
another. - Living cells carry out energy transformations.
3
Energy
- Several forms exist
- Chemical energy
- Light energy
- Mechanical energy
- All energy is either
- Kinetic energy- energy of movement
- Alters the state or motion of matter
- Potential energy- stored energy
- Can be stored in chemical bonds, as a
concentration gradient, as electric potential
4
Energy Conversions and Work
Kinetic energy of river is converted to potential
energy by a dam.
Generator converts kinetic energy of released
water to electric energy.
In cells, energy can be stored in chemical bonds.
5
Metabolism
- 2 types of activities
- Anabolic reactions
- Link simple molecules together to make complex
ones - Energy storing process
- Reactions consume energy
- Catabolic reactions
- Break down complex molecules into simpler ones
- Some reactions provide energy for anabolic
reactions - Reactions release energy
- Anabolic and catabolic reactions are often linked
to do biological work.
6
Figure 6.2 The Laws of Thermodynamics
Energy is neither created nor destroyed.
Amount of free energy available to do work is
always less than the amount of original energy)
Free energy decreases and unusable energy
increases. (entropy-measure of the disorder of a
system
7
- Total energy (H) usable energy (G) unusable
energy (S) - Usable energy to do work is called free energy
(G) - In a chemical reaction if ?G is negative- free
energy is released - If ?G is positive- free energy is required
8
- The amount of energy taken up or released is
directly related to the tendency of the reaction
to run to completion (the point at which all
reactants are converted to products.)
9
Chemical reactions release or take up energy
- A spontaneous reaction will go more than halfway
to completion without input of energy. - Spontaneous reactions are called exergonic.
- Release energy
- Non-spontaneous reactions are called endergonic.
- Consume energy
10
?G is negative
?G is positive
11
ATP Transferring Energy in Cells
- All living cells use ATP for capture, transfer
and storage of energy. - Energy currency
- Energy released by reactions is captured in ATP
ATP can then release energy to drive other
reactions.
12
- Why ATP?
- Releases a relatively large amount of energy when
hydrolyzed - Free energy of the P-O bond between phosphate
groups is much higher than the energy of the H-O
bond after hydrolysis - It takes energy to get phosphate groups near
enough together to link them to make ATP from
ADP. - Can phosphorylate many different molecules
13
ATP hydrolysis releases energy
- Structure of ATP
- Adenine bonded to ribose, carbon 5 of ribose has
3 phosphate groups attached. - ATP can hydrolyze to form ADP plus an inorganic
phosphate ion (Pi) - ATP H20 ----? ADP Pi
14
Figure 6.5 ATP (Part 1)
15
Formation of ATP
- Making ATP from ADP involves overcoming repulsive
negative charges on the phosphates to be joined. - The energy to do this is stored in glucose or
other fuel molecules. - ADP Pi free energy----? ATP H2O
- Each cell requires millions of molecules of ATP
per second to drive its biological machinery.
16
(No Transcript)
17
Synthesis of glutamine from glutamate and NH4 is
endergonic and must be coupled to exergonic
hydrolysis of ATP.
18
Enzymes Biological Catalysts
- Living cells use biological catalysts (enzymes)
to increase and control rates of chemical
reactions. - A catalyst speeds up a reaction without being
permanently altered. - A chemical reaction will occur spontaneously if
it releases free energy, but it may occur to
slowly to be effective in living cells.
19
- Activation energy
- Energy which must be invested to initiate a
reaction. - This energy is absorbed by reactants to break
chemical bonds. - All reactions have activation energy
requirements.
20
Activation energy is small compared to the
overall change in free energy.
21
Over the Energy Barrier
22
Enzymes
- Heating the reactants may increase their kinetic
energy and thus lower the activation energy. - Not efficient or specific, would speed up all
reactions - Could denature proteins
- Enzymes can lower required energy of activation.
- but they do not initiate reactions that could not
eventually take place on their own.
23
Enzyme and Substrate
E S ? ES ? E P
24
How do enzymes speed up reactions?
- At the active site, enzymes and substrates
interact by breaking old bonds and forming new
ones. - Enzyme may change chemically, but is restored to
its original form after reaction is catalyzed.
25
Enzymes Lower the Energy Barrier
?G is not affected by Ea
26
How do enzymes lower the activation energy?
- 1. Orienting substrates
- Two substrates bound to an enzyme are more likely
to be oriented so that a reaction can occur. - 2. Inducing strain in substrates
- Substrate enters active site of enzyme, and the
substrate shape is changed. Stretching of bonds
makes them more reactive. - 3. Temporarily adding chemical groups to
substrates - Enzymes may transfer hydrogen ions, form covalent
bonds with substrate, may lose or gain electrons
27
Orientation
Substrates are oriented so that they can react.
Physical strain
Enzyme strains the substrate
Chemical change
Enzyme adds charges to the substrate
28
Enzyme structure and size
- The active site of an enzyme is usually small.
- 6-12 amino acids
- The whole enzyme is usually composed of hundreds
of amino acids. - The active site is the site where the specific
substrate binds. - Induced fit-
- Many enzymes change their structure when they
bind their substrates - Induced fit brings reactive side chains together
from the active site into alignment with the
substrate.
29
Glucose ATP ------? glucose-6-phosphate ADP
hexokinase
Hexokinase changes shape when substrate binds.
2nd substrate is ATP
Enzyme-hexokinase
Empty active site
Water is excluded from active site.
Shape changes result in an induced fit, improving
the catalytic activity of the enzyme.
30
Non-protein molecules associated with enzymes
- Some enzymes require the presence of other
non-protein molecules in order to function - Cofactors
- Inorganic ions copper ions, zinc ions and iron
ions. - Bind to the enzyme and are essential to function
of some enzymes - Coenzymes
- Small carbon-containing molecules which
temporarily bind to enzymes - Must collide with enzyme and bind to active site
- Prosthetic groups
- Molecular groupings permanently bound to enzymes
31
Metabolic pathways are regulated by enzymes
- Burning of glucose would be an inefficient way to
extract energy from its bonds - Step-by-step pathways catalyzed by enzymes allow
the energy to be extracted in a form that is
usable by cells.
32
Metabolism is organized into pathways
- A-----?B------?C-----?D
- Each step is catalyzed by enzymes.
- One enzyme converts A to B a second enzyme
converts B to C, and so forth. - Pathways can be anabolic or catabolic.
33
Regulation of enzyme activity
- Enzyme activity can be inhibited by natural and
artificial inhibitors. - Natural-regulate metabolism
- Artificial
- Treat disease
- Kill pests
- Study how enzymes work
- DIPF inhibits an enzyme essential for propagation
of nerve impulses - DIPF is a nerve gas related to Sarin
34
- Irreversible inhibitors
- Occurs when inhibitor destroys the enzymes
ability to interact with substrate - Aspirin permanently inactivates cyclooxygenase
- Reversible inhibitors- more likely to occur in
nature - Reversible inhibitors involved in regulation of
metabolic pathways.
35
- Competitive inhibitors
- Bind to active site of enzyme
- Compete with the natural substrate for the active
site - Enzymes function is disabled as long as the
protein remains bound. - Reversible
36
(No Transcript)
37
Figure 6.18 Reversible Inhibition (Part 2)
Inhibitor of enzyme succinate dehydrogenase
38
- Non-competitive inhibitors
- Inhibitor binds at a site distinct from the
active site - Binding causes as conformational change in the
active site so that substrate cannot bind (or
cannot bind as well). - Reversible
39
Inhibitor binding may change shape of enzyme so
that substrate no longer fits.
40
Figure 6.18 Reversible Inhibition (Part 4)
Isoleucine binds to enzyme away from the active
site, but enzyme is altered so that it cannot
interact with substrate.
41
Allosteric enzymes
- Allo- different, stery- shape
- Enzymes may exist in more than one possible
shape. - Allosteric enzymes
- 1. Binding of inhibitor induces enzyme to change
shape, or, more commonly, - 2. Enzymes already exist in cell in different
shapes. - Active form
- Inactive form
- Forms can interconvert, regulated by allosteric
enzymes. - Positive regulators stabilize active form of
enzyme. - Negative regulators stabilize inactive form.
42
Figure 6.19 Allosteric Regulation of Enzymes
43
Figure 6.19 Allosteric Regulation of Enzymes
Conformational change
Inactive form
Active form
The enzyme switches back and forth between the
two forms. They are in equilibrium.
44
Figure 6.19 Allosteric Regulation of Enzymes
Conformational change
Inactive form
Active form
45
Figure 6.19 Allosteric Regulation of Enzymes
Allosteric regulation
Inactive form
When the enzyme is in its inactive form, the
allosteric sites on the regulatory subunits can
accept inhibitor.
46
Figure 6.19 Allosteric Regulation of Enzymes
Allosteric regulation
Inactive form
47
Figure 6.19 Allosteric Regulation of Enzymes
Allosteric regulation
Active form
When the enzyme is in its active form, the active
sites on the catalytic subunits can accept
substrate.
48
Figure 6.19 Allosteric Regulation of Enzymes
Cooperativity
Once a site is filled with a substrate or
inhibitor, binding at a second site of the same
type is favored.
49
Figure 6.19 Allosteric Regulation of Enzymes
Cooperativity
50
Enzymes are affected by their environment
- Enzymes are highly sensitive to
- Temperature
- pH
51
pH Affects Enzyme Activity
52
Temperature Affects Enzyme Activity
53
Allosteric effects regulate metabolism
- End-product or feedback inhibition
- First step in a metabolic pathway is called the
commitment step. - What if the cell has no need for the end-product?
- The final product may allosterically inhibit the
enzyme that catalyzes the commitment step.
54
Inhibition of Metabolic Pathways