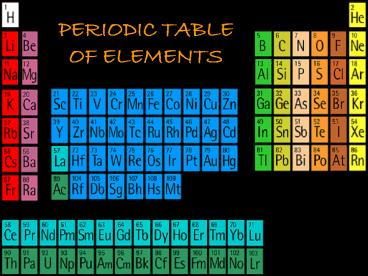
PERIODIC TABLE OF ELEMENTS - PowerPoint PPT Presentation
1 / 53
Title:
PERIODIC TABLE OF ELEMENTS
Description:
The glass tube contains helium gas at low pressure. ... battery, lithium ion, can be recharged and it is often used to power camcorders. ... – PowerPoint PPT presentation
Number of Views:91
Avg rating:3.0/5.0
Title: PERIODIC TABLE OF ELEMENTS
1
PERIODIC TABLE OF ELEMENTS
2
The first element in the periodic table, a light,
highly flammable gas that combines with oxygen to
make water.
3
Ampoule of heavy water. This ampoule contains
0.75ml of deuterium oxide H2O2, better known as
heavy water. It looks identical to ordinary water
but has a density of 1.11 g/cm3 rather than 1.
4
The second element in the periodic table, a
lighter than air, inert gas often used to fill
balloons.
Discharge tube. The glass tube contains helium
gas at low pressure. When 6000 volts of
electricity is passed through the tube, the
helium atoms ionize and give out light producing
a beautiful peachy glow. Helium is the first of
the unreactive noble gases. This tube runs the
hottest of the five discharge tubes and for that
reason you can see a thermal sensor at the back
of the cube that turns off the power if the
temperature rises too high.
5
Soft, light, silvery-white and the first alkali
metal (group1). Lithium reacts vigorously with
water and tarnishes rapidly in air forming both
an oxide and a nitride.
6
Chunks floating in oil. Yes floating! Lithium is
so light that it actually floats on oil. It also
floats on water, but since it also reacts
violently with water, this would not be a good
choice for long term storage. The "air" above the
oil in the ampoule is actually argon. This highly
inert gas prevents oxidation. The lithium was cut
with a knife under oil to reveal its shiny
silvery surface.
7
Lithium batteries. Lithium is an attractive
metal for battery designers because it is very
light, very reactive (hence it stores a lot of
energy) and not too expensive. Batteries based on
alkaline technology are cheaper, so lithium
batteries tend to be used for devices where their
higher power to weight ration are at a premium,
notably cameras. One type of lithium battery,
lithium ion, can be recharged and it is often
used to power camcorders.
8
Bipolar disorder pills. Lithium salts have a
remarkable ability to affect the mind by altering
the chemical state of nerve cells in the brain.
The carbonate and acetate are prescribed for
manic depression and help to limit the extremes
of mental state that are a feature of this
condition. The sulfate and chloride are more
often prescribed as straight anti-depressants.
The bromide finds use as a sedative.
9
A light, silver-white, lustrous metal that is
resistant to corrosion but very toxic, especially
when breathed in as a powder.
10
Lumps. These are solid lumps of beryllium metal,
probably produced by dropping molten Be into
water. They are free of dust and very hard which
is good from a safety point of view as any powder
which might be breathed in must be strictly
avoided. They feel incredibly light and make a
nice clinking noise when hit together.
11
High voltage insulator. Beryllium oxide, also
known as beryllia, has a very high melting point
and is an excellent electrical insulator,
Surprisingly though it is very good conductor of
heat, almost on a par with some metals. These are
useful properties which in this case are deployed
in a high voltage electrical insulator. There is
a label on the device warning that it should not
be machined or broken due to the potential
toxicity of the dust..
12
Beryl. Beryl, a beryllium aluminium silicate, is
a common mineral but its gem varieties are highly
prized. Emerald is the green variety of beryl,
colored by impurities of chromium and vanadium.
Today most noted emeralds originate in Colombia
but ancient emeralds all came from mines south of
El Kassir in Nubia, which were worked from
approximately 3000BC. Cleopatra was reputed to
have had a Nubian emerald engraved with her own
portrait. This specimen is from the Russian
Urals.
13
Emerald. This emerald specimen displays the
clarity and rich green color for which Colombian
emeralds are so prized. The Colombian emerald
mines were worked by native peoples long before
they were taken over by the Spanish in 1537.
Other gem varieties of beryl include aquamarine
(blue-green), bixbite (a red beryl found
exclusively in the Wah Wah Mountains in Utah).
14
A non-metal which exists in several forms
including a brown powder and a hard, dark, shiny
crystal.
15
Crystalline chunks. Boron is most easily
obtainable as a fine brown powder. This is
somewhat flammable (boron powder is an ingredient
of solid rocket fuel). Here boron in a more
attractive solid crystalline form. It is produced
by the thermal decomposition of boron halides at
high temperature.
16
The non-metal on which all living chemistry
depends. Carbon exists in multiple forms
diamond, graphite.
17
Machined graphite block. This display is
machined from a solid block of graphite. It is an
excellent material to mill being easily cut but
capable of taking fine detail. All the dust does
tend to make a mess of the shop however.
18
Levitating graphite. The carbon here is the thin
square of graphite floating above the golden
magnets. There are four of them, made of
neodymium and oriented so that the N and S poles
alternate. Because this type of graphite is a
magnetic material, an opposite magnetic field is
produced and this causes a repulsion force that
keep the feather light square suspended.
19
Diamond. It came as a great surprise when the
great French chemist Antoine Lavoisier
demonstrated in a costly experiment in 1772 that
when you burn diamonds you make carbon dioxide
that is indistinguishable from the product of
burning coal. The difference between coal and
diamond is that in the latter, the carbon atoms
form a 3D network of strong covalent bonds with
one another to form a regular tetrahedral
lattice. In coal and charcoal, by contrast, the
bonding is more random and the resulting material
has much less strength. The rough diamond here is
a natural specimen that was formed a great
temperatures and pressure deep underground.
20
Coal
21
The mainly inert gas which makes up around 78 of
the earth's atmosphere but which also plays a
vital role in the chemistry of living things.
22
A reactive gas that makes up around 20 of the
earth's atmosphere and which reacts with other
elements to form many of the rocks in the crust.
23
The most reactive element in the periodic table.
A pale yellow gas and the first of the halogens.
24
Teflon thread seal tape. Teflon is chemically
similar to the hydrocarbon chains in oil and
plastics and people, with the hydrogen atoms
replaced by fluorine. While hydrocarbons are
mostly carbon, teflon is mostly fluorine because
an atom of fluorine weighs about 19 times more
than an atom of hydrogen. Because fluorine is so
dangerous and difficult to store, and because
teflon is about 75 fluorine by weight, it makes
a good substitute for the real thing in element
collections.
25
Fluorite. Fluorite or fluorspar is a very common
mineral and is named after its fluorine content.
It forms cubic crystals. Fluorite is
subtranslucent to transparent with a vitreous
lustre and is found in a wide variety of colors
colorless, white, yellow, green, blue, red,
purple, pink, brown and black, sometimes more
than one color within the same crystal. Fluorite
is also noted for changes of color depending on
whether light is passing through it or falling
upon it. Some fluorites also exhibit a phenomenon
known as daylight fluorescence, changing color
and glowing under sunlight as opposed to
artificial light.
26
The second member of the noble gases, highly
inert. Widely used as a source of beautiful
illumination when excited by an electric
discharge.
27
Discharge tube. The glass tube bent into the
shape of the letters "Ne" contains pure neon gas
at very low pressure. When 6000v is applied to
the tube, a low current of 20 mA or so causes the
neon atoms to glow with a characteristic orange
red light that is a familiar night time sight in
every town and city. Comparing the brightness of
the discharge with the other noble gases, you can
see why neon is a popular choice.
28
A soft, silvery-white, very light metal which
reacts explosively with water and which rapidly
tarnishes in air
29
This ampoule is filled with pieces of sodium that
were freshly cut under mineral oil to prevent
oxidation. The space at the top is filled with
argon gas to exclude oxygen.
30
Rock salt. It is one of the wonders of chemistry
that two of the most reactive and dangerous
elements (the explosive metal sodium and the
choking gas chlorine) can be combined together to
produce one of the safest and most innocuous
substances common salt!
31
A soft, silvery-white, light metal which burns in
air giving out an intense light. Magnesium is
fairly reactive
32
Cylinder. Magnesium is one of the least dense
metals and the cylinder weighs less than one
tenth of the similar tungsten cylinder that can
be found three places down the periodic table and
four places to the right. The surface has been
sprayed with a thin transparent lacquer to help
prevent oxidation.
33
Ribbon. Magnesium ribbon can be lit with a
match, and will burn with a brilliant, blindly
bright white light, a fact often demonstrated in
high school chemistry labs
34
Camp fire starter. The fact that magnesium burns
very hotly can be used to start camp fires using
this device available for a few dollars at any
Walmart. The idea is to use your hunting knife to
shave off some curls of magnesium from the block,
then strike the back of the knife along the black
flint rod to light the curls. The block is
perfectly safe to carry around because in solid
block form, magnesium is actually very difficult
to ignite. It's only in thin strips or powder
form that it becomes highly flammable.
35
A shiny, light yet strong metal which is used
extensively in industry. It is quite reactive but
protected by a thin transparent layer of oxide
36
I-Beam. Aluminum can be used as a structural
metal, for example in I-beams like this one. The
fact that iron is used much more often is
strictly due to its lower cost and easier
welding, in virtually all other ways aluminum is
superior. Bridges made out of aluminum would
never rust, but they would cost many times as
much to build.
37
Coins. Aluminum coins have a rather cheap feel
to them They definitely give off an air of not
being worth much, which perhaps explains why
aluminum is not used for anything other than the
most worthless coins, like these Japanese yen
coins (worth about a penny, plus or minus,
depending on the exchange rate).
38
Makes up 28 of the earth's crust and is used
extensively in the glass and semi-conductor
industries. A dark, shiny, hard crystalline
semi-metal.
39
Boule top. It is the top of a large crystal of
silicon that has been drawn slowly out of a melt
in the first stage of producing silicon chips.
The result is a long cylinder of ultra-pure
silicon called a boule that is then cut into the
thin wafers you can also see in this cube. The
piece here is the very top of the boule which
cannot be cut up into wafers as it narrows to a
point. Normally it is put back into the melt and
recycled.
40
Wafers. These are beautifully polished wafers of
pure silicon cut from a boule. One has been
photolithographically engraved with the
microscopic circuit patterns that turns it into
integrated circuits (ICs). This is a complex
process that involves building up and then
etching away layers of substrate , conductors and
semi-conductors to create the transistors and the
pathways that form the IC. These are quite small
Modern plants handle 12" diameter wafers and 15"
ones are in the works.
41
Diced chips. A wafer gets cut up into individual
chips like these, which then get mounted in
plastic or ceramic cases and wired up to rows of
pins, forming a complete integrated circuit ready
to be mounted on a circuit board.
42
A non-metal which exists in several forms or
allotropes including a waxy, highly toxic white
form and a less reactive, non-toxic red powder
43
Red phosphorous. These grains of red
phosphorous are the only form of pure phosphorus
that is readily found. It is stable at ordinary
temperatures and much less toxic than white
phosphorus. It is produced by heating the latter
for several days at several hundred degrees
Centigrade
44
White phosphorous. It is a hard waxy substance,
pure white when fresh but yellow as it ages and
slowly converts to the red form. White
phosphorous is an exciting substance that glows
in the dark if just a trace of oxygen is present
and which if allowed to dry spontaneously bursts
into flame producing prodigious amounts of white
phosphorous pentoxide smoke. It is usually stored
under water or mineral oil.
45
A vivid yellow non-metal that exists in several
forms, the most common being an amorphous yellow
powder. It oxidizes readily with a pungent smell.
46
Plastic sulfur. This strange shape is what you
get when you allow a thick trickle of molten
sulfur to fall into cold water. Initially, the
pasta like loops are easy to bend and this
property gives rise to the name of this form of
the element plastic sulfur. However over time
the solidified sulfur crystallized and becomes
brittle, making this preserved shape very
fragile.
47
Fish. Although sulfur is not a metal, it can be
melted and cast just like one. In fact for a
brief period in the 1800's molten-sulfur inlaid
wood furniture was popular.
48
Native Sulfur. Sulfur has been known since
ancient times, its name stemming from the Latin
sulfurium. It is most often associated with
volcanic environments but also forms through the
bacterial decomposition of sulfate deposits and
the burning of coal deposits. Native sulfur takes
many forms from beautiful gem-like transparent
crystals to powdery crusts.
49
The second member of the halogens. A heavy,
highly reactive green gas which combines with
many other elements. With sodium it forms common
salt.
50
The commonest noble gas which makes up almost 1
of the earth's atmosphere. Argon is heavy and
used extensively in welding to prevent oxidation.
51
Discharge tube. When several thousand volts are
applied to argon gas at low pressure the atoms
ionize and a current flows causing light to be
emitted at a characteristic mauve wavelength.
52
http//www.element-collection.com/html/installatio
ns.html
53
PERIODIC TABLE OF ELEMENTS