ConcepTest - PowerPoint PPT Presentation
Title:
ConcepTest
Description:
Hold up as many cards as needed. c) You find the energy of the particle to be E1. ... Hold up as many cards as needed. Example: Expectation Value is Average! ... – PowerPoint PPT presentation
Number of Views:159
Avg rating:3.0/5.0
Title: ConcepTest
1
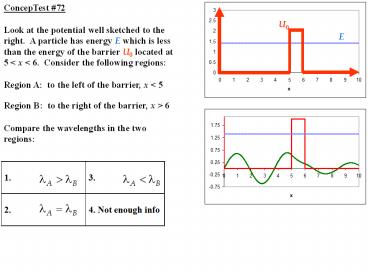
ConcepTest 72 Look at the potential well
sketched to the right. A particle has energy E
which is less than the energy of the barrier U0
located at 5 lt x lt 6. Consider the following
regions Region A to the left of the barrier,
x lt 5 Region B to the right of the barrier, x
gt 6 Compare the wavelengths in the two
regions
E
2
(No Transcript)
3
ConcepTest 73 A system is in the
(superposition) state If you make a
measurement of the system, what is the
probability you would find the system in the
state ? (Alternate What is
?)
Follow-up examples If you make a measurement of
the system, what is the probability of finding
the system in the state ? If you make a
measurement of the system, what is the
probability of finding the system in the state
?
4
Example States and Probabilities A
system is in the (superposition) state
Determine the probability of finding the
particle to be in the state Alternate wording
Calculate
5
ConcepTest 74 Consider a particle of mass m
trapped in an infinite square well (box) of
length L. The state , where
, represents a stationary or pure
state of the particle, with energy . The
particle is in the superposition state
.
a) What is the energy of the superposition
state? Hold up as many cards as needed. b) You
measure the energy of this particle. What value
will you measure? Hold up as many cards as
needed. c) You find the energy of the particle
to be E1. You immediately measure the energy of
this particle again. What value will you
measure? Hold up as many cards as needed.
6
Example Expectation Value is Average!
7
Quantum States Quantum Numbers
mass m, length L1, L2 0 lt x lt L1 0 lt y lt
L2 Pure state Quantum numbers
mass m, length L 0 lt x lt L Pure state
, quantum number
Particle in a 1-D box
Particle in a 2-D box
mass m, length L1, L2 , L3 0 lt x lt L1 0 lt y
lt L2 0 lt z lt L3 Pure state Quantum numbers
Particle in a 3-D box
Each spatial dimension gets one quantum number
Superposition state
8
More than just spatial dimensions
?Particles have INTRINSIC SPIN
QUANTUM SPIN
spin quantum number
Classical Angular Momentum Review
Integer spin quantum number ? BOSONS
examples photons (s 1)
alpha particle(4He nuclei)
Spin Angular Momentum
Earth around axis (day/night) Ball spins on own
axis, etc.
Half - Integer spin quantum number ? FERMIONS
examples electrons, protons, neutrons, (s
½)
Orbital Angular Momentum
Earth around sun (year)
Circular orbits, reference point center of circle