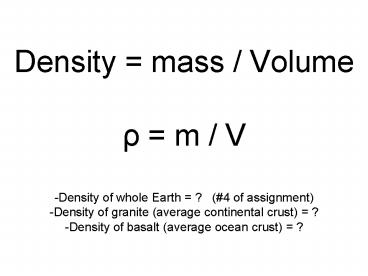Density mass Volume m V Density of whole Earth - PowerPoint PPT Presentation
1 / 28
Title:
Density mass Volume m V Density of whole Earth
Description:
... with definite chemical and physical properties owing to a ... Definite chemical properties. Definite physical properties. Definite crystal structure ... – PowerPoint PPT presentation
Number of Views:205
Avg rating:3.0/5.0
Title: Density mass Volume m V Density of whole Earth
1
Density mass / Volume? m / V-Density of
whole Earth ? (4 of assignment)-Density of
granite (average continental crust) ?-Density
of basalt (average ocean crust) ?
2
Density mass / Volume? m / V-Density of
whole Earth 5.4 g/cm3 (4 of
assignment)-Density of granite (average
continental crust) 2.65 g/cm3 (we got 2.3
g/cm3 )-Density of basalt (average ocean crust)
3.1 g/cm3 (we got 3.1 g/cm3 )-Density of
water 1.0 g/cm3 -Density of lead (Pb) 11.3
g/cm3 -Density of mercury (Hg) 13.5 g/cm3
-Density of Iron (Fe)-Nickel (Ni) meteorites
7.8 g/cm3
3
Lets dissect the Earth!!
- Shall we?
4
Earthquake waves are mechanical waves. They
transmit energy from a source (an Earthquake
focus (more on this later), or a nuclear
explosion) through the Earth. There are 3
kinds. 1.) P-waves Primary waves arrive
first. They are compressional waves. 2.)
S-waves Secondary waves arrive second. They
are shear waves. 3.) Surface waves Travel
along surface. 2 types (more later).
5
P-wave shadow zones
6
S-wave shadow zones. You cant shear a liquid.
7
Fig. 1.03
8
Fig. 2-10, p.18
9
Earth has 1) Fe-Ni solid Inner Core 2)
Fe-Ni-sulfide liquid Outer Core 3) silicate
(Si-O) solid rock Mantle 4) silicate (Si-O)
solid rock Crust. Some evidence 1. Earthquake
shadow zones 2. Mass deficit (?Fe-Ni)(VIC)
(?Fe-Ni-S)(VOC) (3.3-4.5)(VM)
(3.1)(VOceanCrust) (2.65)(VContCrust) 5.4
g/cm3 3. Composition of meteorites (space dust
sampling), stoney (silicate) versus Fe-Ni
meteorites, same as whole Earth composition. 4.
Solid Fe-Ni is peak of nuclear fission-fusion
binding energy curve 5. Earths magnetic field
due to Fe-Ni electromagnetic dynamo. 6. Sulfide
outer core completes Earths sulfur budget
(versus sulfur budget of Venus in its atmosphere,
else a) Greenhouse atmosphere, b) acid
precipitation) 7. Addition of S to Fe-Ni lowers
T-P melting point liquid Outer Core
10
The Earth is differentiated! Differentiation
process by which zones of different composition
are formed by density-driven, gravitational
separation from an initially homogeneous Earth.
11
Divisions of Matter
- Matter substances and mixtures
- Substances elements and compounds
- Elements comprised of atoms (see Periodic table
of elements) - Compounds combinations of elements that are
bonded together. - a. Ionic bonds
- b. Covalent bonds
- Mineral compound
- Mixtures combinations of substances that are
physically separable (crushing, centrifuging,
etc.) - 1. homogeneous e.g. seawater
- 2. heterogeneous Rock heterogeneous mixture
12
(No Transcript)
13
Atomic Theory
- Concept of atom as indivisible piece of matter
comes from the ancient Greeks. - Modern atomic theory revived by English chemist
John Dalton (1766-1844), looking at gas behavior - Dalton was struck by the fact that gases combined
in whole numbers. - Dalton best known for his law of partial
pressures of gases total pressure of a gas
equals the sum of the partial pressures of the
gas components. Note The total pressure may
change after a reaction has run its course. - He knew that, for a given Temperature (T) and
Volume (V), Pressure (P) is proportional to the
amount (mass) of gas. Avogadros law
14
Atom The smallest indivisible piece of an
element that retains its properties. Ernest
Rutherford discovers the nucleus in 1907, that
almost all the mass of an atom is concentrated in
the nucleus. Firing alphas (charged He atoms) at
nucleus, some bounced back!! It was quite the
most remarkable event that has ever happened to
me in my life. It was almost as incredible as if
you fired a 15-inch shell at a piece of tissue
paper and it came back and hit you. Nucleus
contains smaller pieces of divided matter
proton 1 charge, 1amu mass. Atomic number
number of protons neutron 0 charge, 1 amu
mass. electron -1 charge, nearly 0 amu mass.
Mass number sum of protons and neutrons
15
Fig. 2.01
16
Atom The smallest indivisible piece of an
element that retains its properties. Molecule
The smallest indivisible piece of a compound that
retains its properties. Usually refers to
individual small molecules (e.g. individual H2
molecules in a collection of hydrogen gas). The
term molecule does NOT apply to mineral solids,
unless referring to the whole crystal Crystal
A homogeneous solid with a regular (i.e.
repeating) 3D arrangement (i.e. array) of atoms
in a lattice (i.e. regular pattern of points).
The smallest repeating part is called a unit cell.
17
Fig. 2.02
18
Fig. 2.06
19
Fig. A2-3, p.456
20
Fig. 2.07
21
- Mineral
- A naturally occurring, inorganic, solid compound
with definite chemical and physical properties
owing to a definite crystal structure. - Naturally occurring
- Inorganic except for pure C graphite and
diamond, does not involve carbon (coal is not a
mineral) - Compound (note pure metals are called native
elements, e.g. native copper, not minerals). - Definite chemical properties
- Definite physical properties
- Definite crystal structure
22
Dont confuse mineral with ion. In nutrition,
mineral is the same thing as ion. e.g.
Bananas are a good source of essential minerals
like potassium, refers to the K cation. Ion
an electrically charge atom or group of atoms
formed when a neutral atom or group of atoms
gains or loses electrons. A cation is a
positively charged ion that forms when electrons
are lost. An anion is a negatively charged ion
that forms when electrons are gained. Isotope
Atoms of the same element which, because of
different numbers of neutrons in their nucleus,
have different masses (different mass numbers).
23
- Rock
- A mixture of minerals and other substances that
forms the solid part of a planet. Also, a cool
form of music Note coal is a
rock - 3 main types of Rocks.
- Igneous (Fire formed) Rocks formed from melts
(magma and lava). - Sedimentary Rocks formed from cemented broken
fragments (clastic) and/or precipitates
(chemical). - Metamorphic (change form) Rocks formed from
temperature and/or pressure transformation of
other rocks.
24
Fig. 2.10
25
Fig. 2.11
26
Fig. 2.12
27
Rock cycle
28
Sedimentary Rock Terms Bed a layer of sediment
1 cm or more in thickness lamina(e) a layer of
sediment less than 1 cm. in thickness. (adj.
laminated). Bedding (Plane) The surface upon
which sediment was deposited. The surfaces
separating layers of sediment. Stratum a layer
of sedimentary rock (may be comprised of several
beds or laminae). Strata a set of layered
sedimentary rock.