Chapter 5 The Nature of Light - PowerPoint PPT Presentation
1 / 44
Title:
Chapter 5 The Nature of Light
Description:
... hilltop to open a lantern the moment Galileo opened his lantern. ... List the emission of red, green, and blue light in order of increasing wavelength ... – PowerPoint PPT presentation
Number of Views:186
Avg rating:3.0/5.0
Title: Chapter 5 The Nature of Light
1
Chapter 5 The Nature of Light
2
Guiding Ideas
- How fast does light travel (how is this
measured)? c 300,00 km/s (in vacuum), Roemer
1670 Jovian satellite timing over a year - How does light behave like a wave? Interference
effects - How is the light from an ordinary light bulb
different from the light emitted by a neon sign?
Continuous vs. line radiation - How can astronomers measure the surface
temperatures of the Sun, stars, planets? Wiens
Law - What is a photon? Quantum nature of light, energy
prop. to wavelength (duality of wave, particle
picture) - How can astronomers tell what distant celestial
objects are made of? Spectral lines
fingerprints of elements - What are atoms made of? Structure of atoms (Bohr
model) - How does the structure of atoms explain what kind
of light those atoms can emit or absorb? Bohr
model of quantized electron orbits - How can we tell if a star is approaching us or
receding from us? Doppler effect
3
Light travels through empty space incredibly
fast.
- Italian Galileo unsuccessfully attempted to
measure the speed of light by asking an assistant
on a distant hilltop to open a lantern the moment
Galileo opened his lantern.
For hilltops separated by 10 km, time taken for
light is 30 microsec!
4
Light travels through empty space at a speed of
300,00 km/s, called c
- In 1676, Danish astronomer Olaus Røemer
discovered that the exact time of eclipses of
Jupiters moons varied based on how near or far
Jupiter was to Earth. - This occurs because it takes varying amounts of
time for light to travel the varying distance
between Earth and Jupiter.
3108 km
5
Light travels through empty space at a speed of
300,00 km/s, called c
- In 1850, Frenchmen Fizeau and Foucalt showed that
light takes a short, but measurable, time to
travel by bouncing it off a rotating mirror. The
light returns to its source at a slightly
different position because the mirror has moved
during the time light was traveling a known
distance.
6
Light is electromagnetic radiation and is
characterized by its wavelength
- White light is composed of all colors which can
be separated into a rainbow, or a spectrum, by
passing the light through a prism. - Visible light has a wavelength ranging from 400
nm (blue) to 700 nm (red).
7
Although Isaac Newton suggested that light was
made of tiny particles called PHOTONS 130 years
earlier, Thomas Young demonstrated in 1801 that
light has wave-like properties. He passed a beam
of light through two narrow slits which resulted
in a pattern of bright and dark bands on a
stream.
This is the pattern one would expect if light had
wave-like properties.
8
Imagine water passing through two narrow openings
as shown below. As the water moves out, the
resulting waves alternatively cancel and
reinforce each other, much like what was observed
in Youngs Double Slit Experiment.
This is the pattern one would expect if light had
wave-like properties.
9
Today, we understand that light has
characteristics of both particles and waves.
Light behaves according to the same equations
that govern electric and magnetic fields that
move at 300,000 km/s so light is also called
electromagnetic radiation.
Electromagnetic radiation consists of oscillating
electric and magnetic fields. The distance
between two successive wave crests is called the
wavelength and is designated by the letter l.
10
Electromagnetic radiation is produced by stars at
a wide variety of wavelengths in addition to
visible light. Astronomers sometimes describe EM
radiation in terms of frequency, n, instead of
wavelength, l. The relationship is c l x
n Where c is the speed of light, 3 x 108 m/s
11
A dense object emits electromagnetic radiation
according to its temperature.
- WIENS LAW The peak wavelength emitted is
inversely proportional to the temperature. - In other words, the higher the temperature, the
shorter the wavelength (bluer) of the light
emitted.
12
(No Transcript)
13
BLACKBODY CURVES Each of these curves shows the
intensity of light emitted at every wavelength
for an idealized object (called a blackbody)
for several different temperatures. These are
called blackbody curves.
Note that for the objects at the highest
temperature, the maximum intensity is at the
shorter wavelengths and that the total amount of
energy emitted is greatest.
14
Astronomers most often use the Kelvin or Celsius
temperature scales.
In the Kelvin scale, the 0 K point is the
temperature at which there is essentially no
atomic motion is called absolute zero. In the
Celsius scale, this point is 273º C and on the
Fahrenheit scale, this point is -460ºF.
15
(No Transcript)
16
Wiens law and the Stefan-Boltzmann law are
useful tools for analyzing glowing objects like
stars
- Wiens law relates wavelength of maximum emission
for a particular temperature - lmax 0.0029 Tkelvins
- Stefan-Boltzmann law relates a stars energy
output, called ENERGY FLUX, to its temperature - ENERGY FLUX sT4
- ENERGY FLUX is measured in joules per square
meter of a surface per second and s 5.67 X 10-8
W m-2 K-4..
17
A few other useful relationships
- Wiens law relates wavelength of maximum emission
for a particular temperature. - lmax 0.0029 Tkelvins
- Stefan-Boltzmann law relates a stars energy
output, called ENERGY FLUX, to its temperature. - ENERGY FLUX sT4
- ENERGY FLUX is measured in joules per square
meter of a surface per second and s 5.67 X 10-8
W m-2 K-4 - Energy of a photon (in terms of wavelength)
- E h c / l where h 6.625 X 10-34 J s
- or where h 4.135 X 10-15 cV s
- Energy of a photon (in terms of frequency)
- E h n where n is the frequency of light
- These two relationships are called Plancks law.
18
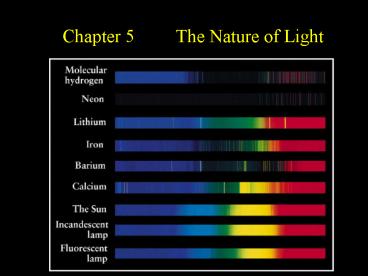
Each chemical element produces its own unique set
of spectral lines.
19
(No Transcript)
20
(No Transcript)
21
The brightness of spectral lines depend on
conditions in the spectrums source.
22
The brightness of spectral lines depend on
conditions in the spectrums source.
- Law 1 A hot opaque body, such as a perfect
blackbody, or a hot, dense gas produces a
continuous spectrum -- a complete rainbow of
colors with without any specific spectral lines.
(This is a black body spectrum.)
23
The brightness of spectral lines depend on
conditions in the spectrums source.
- Law 2 A hot, transparent gas produces an
emission line spectrum - a series of bright
spectral lines against a dark background.
24
The brightness of spectral lines depend on
conditions in the spectrums source.
- Law 3 A cool, transparent gas in front of a
source of a continuous spectrum produces an
absorption line spectrum - a series of dark
spectral lines among the colors of the continuous
spectrum.
25
Kirchhoffs Laws
26
Features of the Suns spectrum created by passing
sunlight through a prism.
27
Emission Line Spectra of A Few Common Elements
28
The Electromagnetic Spectrum
29
Electromagnetic Radiation Radio Waves (TV, ?
1m)
Antenna size 1m
30
But, where does light actually come from?
- Light comes from the movement of electrons in
atoms.
31
Rutherfords Experiment (1915) Showed that Atoms
Are Largely Empty Space!
Alpha particles from a radioactive source are
channeled through a very thin sheet of gold foil.
Most pass through showing that atoms are mostly
empty space, but a few are rejected showing the
tiny nucleus is very massive.
32
An atom consists of a small, dense nucleus
surrounded by electrons (Note Nucleus actually
much smaller)
33
An atom consists of a small, dense nucleus
surrounded by electrons.
- The nucleus contains protons and neutrons
- All atoms with the same number of protons have
the same name (called an element). - Atoms with varying numbers of neutrons are called
isotopes.
- Atoms with a varying numbers of electrons are
called ions.
34
Orbits of electrons
35
Spectral lines are produced when an electron
jumps from one energy level to another within an
atom.
36
(No Transcript)
37
Bohrs formula for hydrogen wavelengths
- 1/l R x 1/N2 1/n2
- N number of inner orbit
- n number of outer orbit
- R Rydberg constant (1.097 X 107 m-1)
- l wavelength of emitted or absorbed photon
38
The wavelength of a spectral line is affected by
the relative motion between the source and the
observer.
39
Doppler Effect Caused by Motion
40
Doppler Shift
- Red Shift The distance between the observer and
the source is increasing. - Blue Shift The distance between the observer and
the source is decreasing. - Dl wavelength shift, Df frequency shift
- lo wavelength if source is not moving
- v velocity of source
- c speed of light
41
Doppler Shift Example
- A spacecraft on its way to Mars transmits a
signal at 100 MHz (1 MHz 106 Hz). It is
received on Earth at 99.99 MHz. How fast is the
spacecraft moving and in which direction?
Since observed frequency is lower, the spacecraft
is moving away from Earth.
42
Chap 5 Key Ideas
- How fast does light travel (how is this
measured)? c 300,00 km/s (in vacuum), Roemer
1670 Jovian satellite timing over a year - How does light behave like a wave? Interference
effects - How is the light from an ordinary light bulb
different from the light emitted by a neon sign?
Continuous vs. line radiation - How can astronomers measure the surface
temperatures of the Sun, stars, planets? Wiens
Law - What is a photon? Quantum nature of light, energy
prop. to wavelength (duality of wave, particle
picture) - How can astronomers tell what distant celestial
objects are made of? Spectral lines
fingerprints of elements - What are atoms made of? Structure of atoms (Bohr
model) - How does the structure of atoms explain what kind
of light those atoms can emit or absorb? Bohr
model of quantized electron orbits - How can we tell if a star is approaching us or
receding from us? Doppler effect
43
PRS Quiz Nature of light and Spectra
- List the emission of red, green, and blue light
in order of increasing wavelength - Blue,green, red
- Red, green, blue
- Blue,red, green
- Green, red, blue
- Xrays travel at what speed? (c is the speed of
light) - Faster than c
- Slower than c
- At exactly c
- Depends on the energy of the x-ray
- The temperature of this room is closest to
- 290K
- 25K
- 273.1K
- 70K
44
- A dilute hot gas (such as a neon beer sign) emits
- Emission line spectrum
- Absorption line spectrum
- Continuous spectrum
- Absorption lines superimposed on continuous
spectrum - Jupiter has a surface temperature of 120K and a
blackbody spectrum which peaks at a wavelength of
30 microns. Plutos blackbody spectrum peaks at
60 microns. What is its surface temperature? - 30K
- 60K
- 120K
- 240K
- The Doppler effect is a change of wavelength
caused by - Gravitational fields between emitter and observer
- Dilute hot gases in the path of the light
- Magnetic fields near the emitter
- Relative motion of the source or observer