Derivatives of Carboxylic Acid - PowerPoint PPT Presentation
Title:
Derivatives of Carboxylic Acid
Description:
Derivatives of Carboxylic Acid carboxylate acid chloride nitrile acid anhydride amide ester Nomenclature of Acid Halides IUPAC: alkanoic acid alkanoyl halide ... – PowerPoint PPT presentation
Number of Views:1299
Avg rating:3.0/5.0
Title: Derivatives of Carboxylic Acid
1
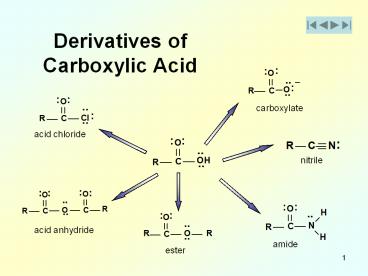
Derivatives of Carboxylic Acid
carboxylate
acid chloride
nitrile
acid anhydride
amide
ester
2
Nomenclature of Acid Halides
- IUPAC alkanoic acid ?? alkanoyl halide
- Common alkanic acid ? alkanyl halide
I 3-aminopropanoyl chloride
I 4-nitropentanoyl chloride
c b-aminopropionyl chloride
c g-nitrovaleryl chloride
I hexanedioyl chloride
c adipoyl chloride
Rings (IUPAC only) ringcarbonyl halide
I benzenecarbonyl bromide
c benzoyl bromide
I 3-cylcopentenecarbonyl chloride
3
Nomenclature of Acid Anhydrides
- Acid anhydrides are prepared by dehydrating
carboxylic acids
acetic anhydride
ethanoic anhydride
ethanoic acid
I butanedioic anhydride
I benzenecarboxylic anhydride
I butanedioic acid
c succinic anhydride
c benzoic andhydride
c succinic acid
Some unsymmetrical anhydrides
I cis-butenedioic anhydride
c maleic anhydride
I benzoic methanoic anhydride
I ethanoic methanoic anhydride
c benzoic formic anhydride
c acetic formic anhydride
4
Nomenclature of Esters
- Esters occur when carboxylic acids react with
alcohols
I phenyl methanoate
I t-butyl benzenecarboxylate
I methyl ethanoate
c phenyl formate
c methyl acetate
c t-butyl benzoate
I dimethyl ethanedioate
I isobutyl cyclobutanecarboxylate
c dimethyl oxalate
c none
I cyclobutyl 2-methylpropanoate
c cyclobutyl a-methylpropionate
5
Nomenclature of Cyclic Esters, Lactones
Cyclic esters, lactones, form when an open
chain hydroxyacid reacts intramolecularly. 5 to
7-membered rings are most stable.
I 4-hydroxybutanoic acid
I 4-hydroxybutanoic acid lactone
c g-hydroxybutyric acid
c g-butyrolactone
- lactone is added to the end of the IUPAC acid
name. - olactone replaces the ic acid of the common
name and hydroxy is dropped but its
locant must be included.
I 5-hydroxypentanoic acid lactone
I 4-hydroxypentanoic acid lactone
c d-valerolactone
c g-valerolactone
I 3-hydroxypentanoic acid lactone
c b-valerolactone
I 6-hydroxy-3-methylhexanoic acid lactone
c b-methyl-e-caprolactone
6
Nomenclature of Amides
1 amide
3 amide N,N-disubstituted amide
2 amide N-substituted amide
- 1 amides alkanoic acid amide ??
alkanamide - a ring is named ringcarboxamide
I butanamide
I p-nitrobenzenecarboxamide
I 3-chlorocyclopentanecarboxamide
c butyramide
c p-nitrobenzamide
c none
- 2 and 3 amides are N-substituted amides
I N-phenylethanamide
c N-phenylacetamide
I N,2-dimethylpropanamide
c acetanilide
c N,a-dimethylpropionamide
I N-ethyl-N-methylcyclobutanecarboxamide
c none
7
Nomenclature of Cyclic Amides, Lactams
Cyclic amides, lactams, form when an open chain
aminoacid reacts intramolecularly. 5 to
7-membered rings are most stable.
I 4-aminobutanoic acid
I 4-aminobutanoic acid lactam
c g-aminobutyric acid
c g-butyrolactam
- lactam is added to the end of the IUPAC acid
name. - olactam replaces the ic acid of the common
name and amino is dropped but its locant
must be included.
I 3-amino-2-bromopropanoic acid lactam
c a-bromo-b-propionolactam
I 5-aminohexanoic acid lactam
c d-caprolactam
I 4-amino-3-methylbutanoic acid lactam
c b-methyl-g-butyrolactam
8
Nomenclature of Nitriles
Nitriles are produced when 1 amides are
dehydrated with reagents like POCl3
- IUPAC alkane nitrile ?? alkanenitrile
- IUPAC rings ringcarbonitrile
- Common alkanic acid onitrile ?
alkanonitrile
I 4-iodobutanenitrile
I p-thiobenzenecarbonitrile
I ethanenitrile
c g-iodobutyronitrile
c p-mercaptobenzonitrile
c acetonitrile
I 3-methoxycyclohexanecarbonitrile
I 2-cyanocyclopentanecarboxylic acid
c none
c none
9
Nomenclature Practice Exercise
I bromomethyl ethanoate
I cyclobutanecarbonitrile
I sodium ethanoate
c bromomethyl acetate
c sodium acetate
c none
I 3-bromo-N-methylpentanamide
c b-bromo-N-methylvaleramide
I pentanedioic anhydride
I 3-oxobutanoyl chloride
c glutaric anhydride
c b-oxobutyryl chloride
I 6-amino-6-chlorohexanoic acid lactam
I 2-ethyl-5-hydroxypentanoic acid lactone
c e-chloro-e-caprolactam
c a-ethyl-d-valerolactone
10
Relative Reactivity of Carbonyl Carbons
- Nucleophiles (electron donors), like OH-, bond
with the sp2 hybridized carbonyl carbon. - The order of reactivity is shown.
11
Nucleophilic Addition to Aldehydes and Ketones
- Recall that electron donors (Nu -s) add to the
electrophilic carbonyl C in aldehydes and
ketones. The CO p bond breaks and the pair of
electrons are stabilized on the electronegative O
atom. - R (alkyl groups) and hydrogens (H) bonded to the
CO carbon remain in place. R- and H- are too
reactive (pKb of 40 and -21). R and H are not
leaving groups, so the carbonyl group becomes an
alkoxide as the sp2 C becomes a tetrahedral sp3
C.
tetrahedral alkoxide with sp3 carbon.
- A second addition of a nucleophile cannot occur
since alkoxides are not nucleophilic. The
reaction is usually completed by protonation of
the alkoxide with H3O forming an alcohol. This
later reaction is simply an acid/base reaction. - The characteristic reaction of aldehydes and
ketones is thus nucleophilic addition.
12
Nucleophilic Acyl Substitution in Acid
Derivatives
- Carboxylic acid derivatives commonly undergo
nucleophilic substitution at the carbonyl carbon
rather than addition. The first step of the
mechanism is the same. - The CO p bond breaks and the pair of electrons
are stabilized on the electronegative O atom. A
tetrahedral alkoxide is temporarily formed.
Chlorine is a fair leaving group.
sp2 carbonyl reforms
alkoxide C js sp3
sp2 carbonyl C
- In carboxylic acid derivates, one of the groups
that was bonded to the carbonyl C is a leaving
group. When this group leaves, the sp3
tetrahedral alkoxide reverts back to an sp2 CO
group. Thus substitution occurs instead of
addition. - In many cases, the substitution product contains
a carbonyl that can react again.
Note that because the CO group reforms, the
nucleophile can react a second time.
13
Nucleophilic Acyl Substitution in Acid Derivatives
- In carboxylic acid derivatives, the acyl group
(RCO) is bonded to a leaving group (-Y).
Draw the mechanism.
- The leaving group (-Y) becomes a base (Y-) .
The acid derivative is reactive If the base
formed is weak (unreactive). Weak bases are
formed from good leaving groups. - For the carboxylic acid derivatives shown, circle
the leaving group. Then draw the structure of
the base formed, give its pKb, and describe it as
a strong or weak base.
acid derivative leaving group pKb strength as base
21
non basic
weak base
9
-2
strong base
-21
v. strong base
14
Nucleophilic Acyl Substitution in Acid Derivatives
- We will study the reaction of only a few
nucleophiles with various carboxylic acid
derivatives and we will see that the same kinds
of reactions occur repeatedly.
- Hydrolysis Reaction with water to produce a
carboxylic acid - Alcoholysis Reaction with an alcohol to produce
an ester - Aminolysis Reaction with ammonia or an amine to
produce an amide - Grignard Reaction Reaction with an
organometallic to produce a ketone or alcohol - Reduction Reaction with a hydride reducing
agent to produce an aldehyde or alcohol
Draw the structures of the expected products of
these nucleophilic substitution reactions, then
circle the group that has replaced the leaving
group (-Y)
hydrolysis
alcoholysis
aminolysis
Grignard reduction
hydride reduction
15
Nucleophilic Acyl Substitution of Carboxylic Acids
- Nucleophilic acyl substitution converts
carboxylic acids into carboxylic acid
derivatives, i.e., acid chlorides, anhydrides,
esters and amides.
NH3, D, -H2O
SOCl2
amide
acid chloride
ROH H
D -H2O
acid anhydride
ester
16
Conversion of Carboxylic Acids to Acid Halides
- The S atom in SOCl2 is a very strong
electrophile. S is electron deficient because it
is bonded to 3 electronegative atoms (Cl and O).
Cl is a leaving group. - The hydroxyl O atom in a carboxylic acid has non
bonded pairs of electrons, making it a
nucleophile. This O atom bonds with S (replacing
a Cl) and forming a chlorosulfite intermediate.
The chlorosulfite group is a very good leaving
group. It is easily displaced by a Cl- ion via an
SN2 mechanism yielding an acid chloride. - Use curved arrows to draw the initial steps of
the mechanism shown below.
- PBr3 will substitute Br for OH converting a
carboxylic acid to an acid bromide - Draw and name the products of the following
reactions.
I p-methylbenzenecarbonyl chloride
c p-methylbenzoyl chloride
I ethanoyl chloride
c acetyl chloride
17
Conversion of Carboxylic Acids to Acid Anhydrides
- High temperature dehydration of carboxylic acids
results in two molecules of the acid combining
and eliminating one molecule of water.
acetic anhydride
ethanoic anhydride
ethanoic acid
- Cyclic anhydrides with 5 or 6-membered rings are
prepared by dehydration of diacids.
I butanedioic acid
I butanedioic anhydride
c succinic acid
c succinic anhydride
- Draw a reaction showing the preparation of
cyclohexanecarboxylic anhydride.
18
Conversion of Carboxylic Acids to Esters
- Two methods are used SN2 reaction of a
carboxylate and Fischer Esterification - SN2 reaction of a carboxylate with a methyl
halide or 1? alkyl halide is straightforward.
2? and 3? alkyl halides are not used because
carboxylate is only a fair nucleophile and is
basic enough (pKb 9) that elimination of HX
from the alkyl halide will compete with
substitution. The carboxylate will be protonated
and the alkyl halide eliminates HX becoming an
alkene.
E2
sodium propionoate
isobutylene
I 5-bromopentanoic acid
I 5-hydroxypentanoic acid lactone
I sodium 5-bromopentanoate
c d-bromovaleric acid
c d-valerolactone
c sodium d-bromovalerate
19
Conversion of Carboxylic Acids to Esters
20
Conversion of Carboxylic Acids to Esters
- Fischer Esterification (RCOOH ? RCOOR)
Esters are produced from carboxylic acids by
nucleophilic acyl substitution by a methyl or 1º
alcohol. Heating the acid and alcohol in the
presence of a small quantity of acid catalyst
(H2SO4 or HCl (g)) causes ester formation
(esterification) along with dehydration. The
equilibrium constant is not large (Keq 1) but
high yields can be obtained by adding a large
excess of one of the reactants and removing the
H2O formed. The reaction is reversible. A large
excess of H2O favors the reverse reaction. Bulky
(sterically hindered) reagents reduce yields.
Since alcohols are weak nucleophiles, acid
catalyst is used to protonate the carbonyl oxygen
which makes the carbonyl C a better electrophile
for nucleophilic attack by ROH. Proton transfer
from the alcohol to the hydroxyl creates a better
leaving group (HOH). Learn the mechanism since
it is common to other reactions.
- The net effect of Fischer esterification is
substitution of the OH group of a carboxylic
acid with the OR group of a methyl or 1 alcohol.
21
Conversion of Carboxylic Acids to Esters
- Draw and name the products of the following
reactions.
I diethyl propanedioate
I propanedioic acid
c diethyl malonate
c malonic acid
cyclopentylmethyl benzoate
- Draw the reagents that will react to produce the
following ester.
Why will an SN2 reaction of a carboxylate and an
alkyl halide not work here?
I isopropyl 2-methylpropanoate
Isopropyl bromide is a 2 alkyl halide and would
undergo an E2 rather than SN2 reaction.
c isopropyl isobutyrate
- Draw the complete mechanism for Fischer
esterification of benzoic acid with methanol.
22
Conversion of Carboxylic Acids to Amides
- Amides are difficult to prepare by direct
reaction of carboxylic acids with amines (RNH2)
because amines are bases that convert carboxylic
acids to non electrophilic carboxylate anions and
themselves are protonated to non nucleophilic
amine cations, (RNH3)
- High temperatures are required to dehydrate these
quaternary amine salts and form amides. This is
a useful industrial method but poor laboratory
method. - In the lab amides are often prepared from acid
chloride after converting the carboxylic acid to
the acid chloride.
Proton (H) acceptor
- Explain why methylamine is a Bronsted base.
- Explain why methylamine is a Lewis base.
- Explain why methylamine is not an Arrhenius base
Electron pair donor
Has no OH- group
23
Synthesis Problems Involving Carboxylic Acids
- Write equations showing how the following
transformations can be carried out. Form a
carboxylic acid at some point in each question.
24
Chemistry of Acid Halides
- In the same way that acid chlorides are produced
by reacting a carboxylic acid with thionyl
chloride (SOCl2), acid bromides are produced by
reacting a carboxylic acid with phosphorus
tribromide (PBr3).
Reactions of Acid Halides Acid halides are among
the most reactive of the carboxylic acid
derivatives and are readily converted to other
compounds. Recall that acid chlorides add to
aromatic rings via electrophilic aromatic
substitution (EAS) reactions called
Friedel-Crafts Acylation with the aid of
Friedel-Crafts catalysts.
25
Chemistry of Acid Halides
- Draw a reaction showing how propylbenzene can be
produced by a Friedel Crafts acylation reaction.
I 1-phenyl-1-propanone
c ethyl phenyl ketone
- Most acid halide reactions occur by a
nucleophilic acyl substitution mechanism. The
halogen can be replaced by -OH to produce an
acid, -OR to produce an ester, -NH2 to produce an
amide. Hydride reduction produces a 1? alcohol,
and Grignard reaction produces a 3? alcohol.
26
Hydrolysis Conversion of Acid Halides into Acids
- Acid chlorides react via nucleophilic attack by
H2O producing carboxylic acids and HCl.
- Tertiary amines, such as pyridine, are sometimes
used to scavenge the HCl byproduct and drive the
reaction forward. 3º amines will not compete
with water as a nucleophile because their
reaction with acid halide stops at the
intermediate stage (there is no leaving group).
Eventually, water will displace the amine from
the tetrahedral intermediate, regenerating the 3º
amine and forming the carboxylic acid.
- Draw the mechanism of the reaction of
cyclopentanecarbonyl chloride with water.
27
Alcoholysis Conversion of Acid Halides into
Esters
- Acid chlorides react with alcohols producing
esters and byproduct HCl by the same mechanism as
hydrolysis above. - Draw and name the products of the following
reaction.
I isopropyl ethanoate
I ethanoyl chloride
c isopropyl acetate
c acetyl chloride
- Draw the mechanism of the reaction of benzoyl
chloride and ethanol.
- Once again, 3º amines such as pyridine may be
used to scavenge the HCl byproduct or for water
insoluble acid halides, aqueous NaOH can be used
to scavenge HCl since it will not enter the
organic layer and attack the electrophile (thus
it cannot compete with the alcohol as the
nucleophile).
28
Practice on Synthesis of Esters
- Write equations showing all the ways that benzyl
benzoate can be produced. Consider Fischer
esterification, SN2 reaction of a carboxylate
with an alkyl halide, and alcoholysis of an acid
chloride.
- Answer the same question as above but for t-butyl
butanoate
This is the only method that will work.
- Explain why the other methods will fail.
29
Aminolysis Conversion of Acid Halides into Amides
- Acid chlorides react rapidly with ammonia or 1?
or 2? but not 3? amines producing amides. Since
HCl is formed during the reaction, 2 equivalents
of the amine are used. 1 equivalent is used for
formation of the amide and a second equivalent to
react with the liberated HCl, forming an ammonium
chloride salt. Alternately, the second
equivalent of amine can be replaced by a 3º amine
or an inexpensive base such as NaOH (provided it
is not soluble in the organic layer). Using NaOH
in an aminolysis reaction is referred to as the
Schotten-Baumann reaction.
I N,N-dimethylbenzenecarboxamide
c N,N-dimethylbenzamide
- Write equations showing how the following
products can be made from an acid chloride.
N-methylacetamide
propanamide
30
Reduction of Acid Chlorides to Alcohols with
Hydride
- Acid chlorides are reduced by LiAlH4 to produce
1? alcohols. The alcohols can of course be
produced by reduction of the carboxylic acid
directly. - The mechanism is typical nucleophilic acyl
substitution in which a hydride (H-) attacks the
carbonyl C, yielding a tetrahedral intermediate,
which expels Cl-. The result is substitution of
-Cl by -H to yield an aldehyde, which is then
immediately reduced by LiAlH4 in a second step to
yield a 1? alcohol.
- Draw the reaction and name the product when
2,2-dimethylpropanoyl chloride is reduced with
LiAlH4
I 2,2-dimethyl-1-propanol
c neopentyl alcohol
31
Reduction of Acid Chlorides to Aldehydes with
Hydride
- The aldehyde cannot be isolated if LiAlH4 (and
NaBH4) are used. Both are too strongly
nucleophilic. - However, the reaction will stop at the aldehyde
if exactly 1 equivalent of a weaker hydride is
used, i.e., diisobutylaluminum hydride (DIBAH) at
a low temperature (-78C). - Under these conditions, even nitro groups are not
reduced.
- DIBAH is weaker than LiAlH4. DIBAH is neutral
LiAlH4 is ionic. - DIBAH is similar to AlH3 but is hindered by its
bulky isobutyl groups. - Only one mole of H- is released per mole of
DIBAH.
p-nitrobenzaldehyde
32
Reduction of Acid Chlorides to Alcohols with
Grignards
- Grignard reagents react with acid chlorides
producing 3? alcohols in which 2 alkyl group
substituents are the same. The mechanism is the
same as with LiAlH4 reduction. The 1st
equivalent of Grignard reagent adds to the acid
chloride, loss of Cl- from the tetrahedral
intermediate yields a ketone, and a 2nd
equivalent of Grignard immediately adds to the
ketone to produce an alcohol.
I 2-phenyl-2-propanol
- The ketone intermediate cant be isolated with
Grignard reaction but can be with Gilman reagent
(diorganocopper), R2CuLi. Only 1 equivalent of
Gilman is used at -78C to prevent reaction with
the ketone product. Recall the preparation of
ketones (Ch. 19). This reagent does not react
other carbonyl compounds (although it does
replace halogens in alkyl halides near 0?C)
I 3-methyl-2-butanone
c isopropyl methyl ketone
33
Practice Questions for Acid Chloride Reductions
- Draw the reagents that can be used to prepare the
following products from an acid chloride by
reduction with hydrides, Grignards and Gilman
reagent. Draw all possible combinations.
I ethanoyl chloride
I 1,1-dicyclopentylethanol
I 1-phenyl-1-propanone
c ethyl phenyl ketone
I 2,2-dimethylpropanoyl chloride
I 2,2-dimethyl-1-propanol
I cyclohexanecarbonyl chloride
I cyclohexanecarbaldehyde
34
Preparations of Acid Anhydrides
Preparation of Acid Anhydrides Dehydration of
carboxylic acids as previously discussed is
difficult and therefore limited to a few cases.
acetic anhydride
A more versatile method is by nucleophilic acyl
substitution of an acid chloride with a
carboxylate anion. Both symmetrical and
unsymmetrical anhydrides can be prepared this way.
- Draw all sets of reactants that will produce the
anhydride shown with an acid chloride.
35
Reactions of Acid Anhydrides
The chemistry of acid anhydrides is similar to
that of acid chlorides except that anhydrides
react more slowly. Acid anhydrides react with
HOH to form acids, with ROH to form esters, with
amines to form amides, with LiAlH4 to form 1?
alcohols and with Grignards to form 3? alcohols.
Note that ½ of the anhydride is wasted so that
acid chlorides are more often used to acylate
compounds. Acetic anhydride is one exception in
that it is a very common acetylating agent.
- Write the mechanism for the following reactions
and name all products - aniline with acetic anhydride (2 moles aniline
are needed or use 1 mole aq. NaOH) - cyclopentanol with acetic formic anhydride (the
formic carbonyl is more reactive). - methyl magnesium bromide with acetic propanoic
anhydride (Grignards are not nucleophilic enough
to react with carboxylate by products) - lithium aluminum hydride with acetic formic
anhydride (LiAlH4 is so powerful a nucleophile
that it will reduce even carboxylates).
36
Practice Questions for Acid Anhydrides
- Show the product of methanol reacting with
phthalic anhydride
2-(methoxycarbonyl)benzoic acid
- Draw acetominophen formed when p-hydroxyaniline
reacts with acetic anhydride
N-(4-hydroxyphenyl)acetamide
37
Chemistry of Esters
- Esters are among the most widespread of all
naturally occurring compounds. Most have
pleasant odors and are responsible for the
fragrance of fruits and flowers. Write chemical
formulas for the following esters
Flavor Name Structure
pineapple methyl butanoate
bananas isopentyl acetate
apple isopentyl pentanoate
rum isobutyl propanoate
oil of wintergreen methyl salicylatemethyl 2-hydroxybenzoate)
nail polish remover ethyl acetate
new car smell(plasticizer for PVC) dibutyl phthalate
38
Preparation of Esters
- SN2 reaction of a carboxylate anion with a methyl
or 1? alkyl halide - Fischer esterification of a carboxylic acid
alcohol acid catalyst - Acid chlorides react with alcohols in basic media
39
Reactions of Esters
- Esters react like acid halides and anhydrides but
are less reactive toward nucleophiles because the
carbonyl C is less electrophilic. Both acyclic
esters and cyclic esters (lactones) react
similarly. Esters are hydrolyzed by HOH to
carboxylic acids, react with amines to amides,
are reduced by hydrides to aldehydes, then to
1?alcohols, and react with Grignards to 3?
alcohols.
40
Base Hydrolysis of Esters
- Esters are hydrolyzed (broken down by water) to
carboxylic acids or carboxylates by heating in
acidic or basic media, respectively. - Base-promoted ester hydrolysis is called
saponification (Latin soap-making). Boiling
animal fat (which contains ester groups) in an
aqueous solution of a strong base (NaOH, KOH,
etc.) makes soap. A soap is long hydrocarbon
chain with an ionic end group.
I sodium dodecanoate
c sodium laurate
- The mechanism of base hydrolysis is nucleophilic
acyl substitution in which OH- adds to the ester
carbonyl group producing a tetrahedral
intermediate. The carbonyl group reforms as the
alkoxide ion leaves, yielding a carboxylate.
c potassium laurate
- The leaving group, methoxide (OCH3-), like all
alkoxides, is a strong base (pKb -2). It will
deprotonate the carboxylic acid intermediate
converting it to a carboxylate. The alkoxide,
when neutralized, becomes an alcohol.
41
Acid Hydrolysis of Esters
- Acidic hydrolysis of an ester yields a carboxylic
acid (and an alcohol). The mechanism of acidic
ester hydrolysis is the reverse of Fischer
esterification. The ester is protonated by acid
then attacked by the nucleophile HOH. Transfer
of a proton and elimination of ROH yields the
carboxylic acid. The reaction is not favorable.
It requires at least 30 minutes of refluxing. - Draw the complete mechanism of acid hydrolysis of
methyl cyclopentanecarboxylate.
- Acid hydrolysis of an ester can be reversed by
adding excess alcohol. The reverse reaction is
called Fischer Esterification. Explain why base
hydrolysis of an ester is not reversible.
42
Alcoholysis of Esters
- Nucleophilic acyl substitution of an ester with
an alcohol produces a different ester. The
mechanism is the same as acid hydrolysis of
esters except that that the nucleophile is an
alcohol rather than water. A dry acid catalyst
must be used, e.g., HCl(g) or H2SO4. If water is
present, it will compete with the alcohol as the
nucleophile producing some carboxylic acid in
place of the ester product. - The process is also called Ester Exchange or
Transesterification
dicyclobutyl 1,4-benzenedicarboxylate
dicyclobutyl terephthalate
diethyl 1,4-benzenedicarboxylate
diethyl terephthalate
cyclobutanol
43
Aminolysis of Esters
- Amines can react with esters via nucleophilic
acyl substitution yielding amides but the
reaction is difficult, requiring a long reflux
period. Aminolysis of acid chlorides is
preferred. - Draw the mechanism aminolysis of methyl
isobutyroxide with ammonia.
I 2-methylpropanamide
c a-methylpropionamide
- Write an equation showing how the following amide
can be prepared from an ester.
- Note that the amide intermediate must deprotonate
to form a stable, neutral amide. Thus the amine
must have at least one H. NH3, 1 and 2 amines
will work but not 3.
44
Hydride Reduction of Esters
- Esters are easily reduced with LiAlH4 to yield 1?
alcohols. The mechanism is similar to that of
acid chloride reduction. A hydride ion first
adds to the carbonyl carbon temporarily forming a
tetrahedral alkoxide intermediate. Loss of the
OR group reforms the carbonyl creating an
aldehyde and an OR - ion. Further addition of H
- to aldehyde gives the 1? alcohol. Draw the
mechanism and show all products.
- Draw and name the products.
I 1,4-butanediol
I 4-hydroxybutanoic acid lactone
c none
c g-butyrolactone
- The hydride intermediate can be isolated if DIBAH
is used as a reducing agent instead of LiAlH4. 1
equivalent of DIBAH is used at very low temp.
(-78 ?C).
I 4-hydroxypentanal
I 4-hydroxypentanoic acid lactone
c g-hydroxyvaleraldehyde
c g-valerolactone
45
Grignard Reduction of Esters
- Esters and lactones react with 2 equivalents of
Grignard reagent to yield 3? alcohols in which
the 2 substituents are identical. The reaction
occurs by the usual nucleophilic substitution
mechanism to give an intermediate ketone, which
reacts further with the Grignard to yield a 3?
alcohol.
triphenylmethoxide
methyl benzoate
benzophenone
triphenylmethanol
I 4-hydroxybutanoic acid lactone
4-methyl-1,4-pentanediol
c g-butyrolactone
46
Practice with Esters
- What ester and Grignards will combine to produce
the following
2-phenyl-2-propanol
1,1-diphenylethanol
47
Chemistry of Amides
- Amides are usually prepared by reaction of an
acid chloride with an amine. Ammonia,
monosubstituted and disubstituted amines (but not
trisubstituted amines) all react.
- Amides are much less reactive than acid
chlorides, acid anhydrides, or esters. Amides
undergo hydrolysis to yield a carboxylic acids
plus an amine on heating in either aqueous acid
or aqueous base. - Basic hydrolysis occurs by nucleophilic addition
of OH- to the amide carbonyl, followed by
elimination of the amide ion, NH2-,(a very
reactive base a difficult step requiring
reflux)
I sodium cyclohexanecarboxamide
48
Hydrolysis of Amides
- Acidic hydrolysis occurs by nucleophilic addition
of HOH to the protonated amide, followed by loss
of a neutral amine (after a proton transfer to
nitrogen).
N-methylcyclohexanecarboxamide
cyclohexanecarboxylic acid
5-aminopentanoic acid lactam
d-valerolactam
49
Alcoholysis of Amides (to Esters)
- Alcoholysis of amides occurs by the same acid
catalyzed mechanism as acid hydrolysis except
that the amido group of the amide is replaced
with by an alcohol rather than water. Dry acid,
e.g., HCl(g) or H2SO4 must be used otherwise
water would compete with the alcohol as the
nucleophile producing some carboxylic acid
product in place of an ester. - The reaction will require a long reflux period
because amides are weak electrophiles and
alcohols are weak nucleophiles.
N,N-dimethylcyclopentanecarboxamide
sec-butyl cyclopentanecarboxylate
- Write a mechanism for this reaction. Refer to
acid hydrolysis mechanism if necessary.
50
Hydride Reduction of Amides
- Amides are reduced by LiAlH4. The product is an
amine rather than an alcohol. The amide carbonyl
group is converted to a methylene group (-CO ?
-CH2). This is unusual. It occurs only with
amides and nitriles. Initial hydride attack on
the amide carbonyl eliminates the oxygen. A
second hydride ion is added to yield the amine.
The reaction works with lactams as well as
acyclic amides.
N,N-dimethylcyclopentanecarboxamide
- Write equations showing how the above
transformation can be carried out.
benzoyl chloride
N-methylbenzamide
51
Grignard Reduction of Amides
- Grignards deprotonate 1º and 2º amides and are
not reactive enough to add to the imide ion
product. N-H protons are acidic enough (pKa 17)
to be abstracted by Grignards.
- Write equations showing how the following
transformation can be carried out.
52
Chemistry of Nitriles
- The carbon atom in the nitrile group is
electrophilic because it is bonded to an
electronegative N atom and a ? bond in the
nitrile is easily broken, i.e., as if it were
providing a leaving group.
- Preparation of Nitrile
- Nitriles are easily prepared by SN2 reaction of
cyanide ion (CN-) with methyl halides or a 1?
alkyl halide. 2º alkyl halides also work but
some E2 product also forms. 3º alkyl halides
will result in mostly an alkene (E2) product
instead of a nitrile. (pKb of CN- 4.7)
propanenitrile
bromoethane
ethyl bromide
- Another method of preparing nitriles is by
dehydration of a 1? amide using any suitable
dehydrating agent such as SOCl2, POCl3, P2O5, or
acetic anhydride. Initially, SOCl2 reacts with
the amide oxygen atom and elimination follows.
This method is not limited by steric hindrance.
53
Reactions of Nitriles
- Like carbonyl groups, the nitrile group is
strongly polarized and the nitrile C is
electrophilic. Nucleophiles thus attack yielding
an sp2 hybridized imine anion.
- Nitriles are hydrolyzed by HOH to amides and
subsequently to carboxylic acids, reduced by
hydrides to amines or aldehydes, and by Grignards
to ketones.
54
Hydrolysis of Nitriles into Carboxylic Acids
- Nitriles are hydrolyzed in either acidic or basic
aqueous solution to yield carboxylic acids plus
ammonia or an amine.
- In acid media, protonation of N produces a cation
that reacts with water to give an imidic acid (an
enol of an amide). Keto-enol isomerization of
the imidic acid gives an amide. The amide is
then hydrolyzed to a carboxylic acid and ammonium
ion. It is possible to stop the reaction at the
amide stage by using only 1 mole of HOH per mole
of nitrile. Excess HOH forces carboxylic acid
formation.
55
Hydrolysis of Nitriles into Carboxylate Salts
- In basic media, hydrolysis of a nitrile to a
carboxylic acid is driven to completion by the
reaction of the carboxylic acid with base. The
mechanism involves nucleophilic attack by
hydroxide ion on the electrophilic C producing a
hydroxy imine, which rapidly isomerizes to an
amide. Further hydrolysis yields the carboxylate
salt.
- Show how the following transformation can be
carried out without using a Grignard.
56
Reduction of Nitriles
Alcoholysis of Nitriles doesnt work. Alcohols
are weak nucleophiles and nitriles are weak
electrophiles Aminolysis of Nitriles doesnt
work. Amines are weak nucleophiles and nitriles
are weak electrophiles. Reduction with
Hydrides Reduction of nitriles with 2
equivalents of LiAlH4 gives 1? amines. LiAlH4 is
a very good nucleophile and can break 2 ? bonds
forming a dianion.
- If less powerful DIBAH is used, only 1 equivalent
of hydride can add. Subsequent addition of HOH
yields the aldehyde.
2-methylbenzaldehyde
57
Reduction of Nitriles with Grignards
- Grignards add to nitriles giving intermediate
imine anions which when hydrolyzed yield ketones.
The mechanism is similar to hydride reduction
except that the attacking nucleophile is a
carbanion (R-). Grignards are not as strongly
nucleophilic as LiAlH4 and so can only add once
a dianion is not formed.
1-phenyl-1-propanone
ethyl phenyl ketone
58
Multistep Synthesis Problems
- Write equations to show how the following
transformations can be carried out.