Why are double-network hydrogels so tough? - PowerPoint PPT Presentation
Why are double-network hydrogels so tough?
Why are double-network hydrogels so tough? T. Tominaga1,2, V.R. Tirumala1, P.D. Butler1, E.K. Lin1, H. Furukawa2, J.P. Gong2, Y. Osada2, and W.-L. Wu1 – PowerPoint PPT presentation
Title: Why are double-network hydrogels so tough?
1
Why are double-network hydrogels so tough?
T. Tominaga1,2, V.R. Tirumala1, P.D. Butler1,
E.K. Lin1, H. Furukawa2, J.P. Gong2, Y. Osada2,
and W.-L. Wu1
1NIST, 2Hokkaido University
DMR-0454672
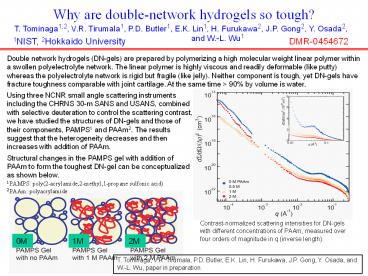
Double network hydrogels (DN-gels) are prepared
by polymerizing a high molecular weight linear
polymer within a swollen polyelectrolyte network.
The linear polymer is highly viscous and readily
deformable (like putty) whereas the
polyelectrolyte network is rigid but fragile
(like jelly). Neither component is tough, yet
DN-gels have fracture toughness comparable with
joint cartilage. At the same time gt 90 by volume
is water.
Using three NCNR small angle scattering
instruments including the CHRNS 30-m SANS and
USANS, combined with selective deuteration to
control the scattering contrast, we have studied
the structures of DN-gels and those of their
components, PAMPS1 and PAAm2. The results suggest
that the heterogeneity decreases and then
increases with addition of PAAm. Structural
changes in the PAMPS gel with addition of PAAm to
form the toughest DN-gel can be conceptualized as
shown below.
1 PAMPS poly(2-acrylamide,2-methyl,1-propane
sulfonic acid) 2 PAAm polyacrylamide
Contrast-normalized scattering intensities for
DN-gels with different concentrations of PAAm,
measured over four orders of magnitude in q
(inverse length).
T. Tominaga, V.R. Tirumala, P.D. Butler, E.K.
Lin, H. Furukawa, J.P. Gong, Y. Osada, and W.-L.
Wu, paper in preparation.
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.