Chemical Structure of Ramipril - PowerPoint PPT Presentation
1 / 26
Title:
Chemical Structure of Ramipril
Description:
Lisinopril. Captopril. Therapie 1995; 50: 131-136. J Hypertens 1991; 9: 1057-1062 ... Fosinopril Lisinopril. Lisinopril Ramipril. Quinapril. Ramipril ... – PowerPoint PPT presentation
Number of Views:893
Avg rating:5.0/5.0
Title: Chemical Structure of Ramipril
1
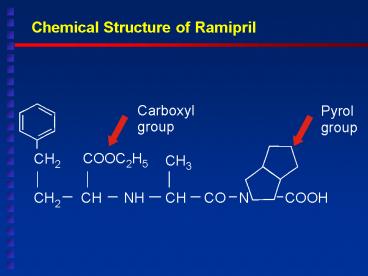
Chemical Structure of Ramipril
2
Chemical Structure
3
Lipophilicity increases drug penetration and
availability at the site of action
Ramiprilat
Enalaprilat
Lipophilic index
Lisinopril
Captopril
Therapie 1995 50 131-136 J Hypertens 1991 9
1057-1062
4
Ramipril has been proven to inhibit tissue ACE
activity in humans
120
99.7
97.5
100
80
60.9
Inhibition ()
60
35
40
20
0
Renal cortex
Heart
Artery
Vein
J Hypertens 1991 9 1057-1062 Am J
Health-Syst Pharm 2000 57 (Suppl 1) S3-S7
5
AIRE (Acute Infarction Ramipril Efficacy) Study
35
Placebo
)
30
(
y
Ramipril
t
i
25
l
2.5-5 mg bd
a
t
r
o
20
M
e
v
15
i
t
a
l
u
10
m
Risk reduction 27
u
Avg follow-up 15 months
C
5
0
0
6
12
18
24
30
Time (Months)
n1986
Lancet 1993 342 821-828
6
AIREX (AIRE Extension) Study
100
n603
l
a
i
90
v
i
v
r
u
s
e
80
v
i
t
a
l
u
70
m
u
c
Relative risk reduction 36
60
Ramipril
Placebo
0
0
1
2
3
4
5
Time from randomisation (years)
Lancet 1997 349 1493-1497
7
HOPE (Heart Outcomes Prevention Evaluation) Study
- Patients gt 55 years with a history of
- - CAD or
- - Stroke or
- - PVD or
- - Diabetes plus at least one other CV risk
factor (hypertension, elevated total cholesterol
levels, low HDL levels, cigarette smoking or
microalbuminuria) - Patients did not have heart failure or LV
dysfunction - 9297 patients received ramipril or placebo
- Ramipril initiated at a dose of 2.5 mg for one
week, 5 mg for the next 3 weeks and then 10 mg. - In addition, all patients received 400 IU of
vitamin E or placebo - Treatment duration 4.5 years
8
HOPE Study Results
Kaplan-Meier Estimates of the Composite Outcome
of Myocardial Infarction, Stroke, or Death from
Cardiovascular Cause
Risk reduction 22
N Engl J Med 2000 342 145-153
9
HOPE Study Results (contd.)
risk reduction
N Engl J Med 2000 342 145-153
10
HOPE Benefits in all subgroups
- Younger than 65 years as well as 65 years and
older - With/without diabetes
- With/without evidence of cardiovascular disease
- With/without hypertension
- With/without microalbuminuria
- Whether or not taking aspirin or other
antiplatelet agents, beta blockers,
lipid-lowering agents or antihypertensive agents - NEJM 2000 342 145-153
11
HOPE Reasons for benefit with ramipril
- Only a small part of the benefit could be
attributed to the reduction in BP (3/2 mmHg) - Inhibition of ACE prevents events related to
ischemia and atherosclerosis, in addition to
those related to heart failure and LV dysfunction - Direct effect on heart and vessels prevention of
vasoconstriction, proliferation of vascular
smooth muscle cells and rupture of plaques,
improving endothelial function, reducing LVH and
enhancing fibrinolysis - NEJM 2000 342 145-153
12
HOPE study Implications
- Ramipril is beneficial in a broad range of
patients who are at high risk of cardiovascular
events - If ramipril is widely used in high-risk
patients, more than one million unnecessary
deaths, heart attacks and strokes could be
prevented worldwide each year - -Dr Salim Yusuf
13
MICRO-HOPE Study Results
Myocardial
infarction,
Overt
stroke or
nephropathy,
cardiovascular
Myocardial
Cardiovascular
dialysis or
Overt
death
Stroke
infarction
death
laser therapy
nephropathy
Total mortality
0
-10
Risk Reduction
-16
-20
-22
-24
-24
-25
-30
-33
-37
-40
Lancet 2000 355 253-259
n3577
14
MICRO-HOPE Study Reasons for benefit
Implications
- Benefits seen in those with and without
hypertension, with or without history of CV
events, or microalbuminuria - Benefits seen in Type 1 and Type 2 diabetes
- Reduction in BP only 2.2/1.4 mmHg
- Benefits due to direct effects on the arterial
wall (vasodilation, anti-trophic effects and
stabilisation of plaque)
Lancet 2000 355 253-259
15
Ramipril in Diabetic Nephropathy (Hypertensives)
PLACEBO
3rd MONTH
6th MONTH
12th MONTH
18th MONTH
5
0
0
Incl Crit DBP95-105 mmHg UAE 300-2000 mg/24h
NS
-4,5
0
-5
s
NS
e
g
-9,1
n
a
-10
h
c
E
A
-15
U
P lt0.05
r
P lt0.05
u
o
-21,4
-20
h
-22,7
-
4
2
e
-25
g
a
-23,1
t
n
P lt0.05
e
-30
c
r
e
P
NITRENDIPINE 20 mg
-35
-33,3
RAMIPRIL
-34,8
P lt0.01
-40
P lt0.01
P lt0.01
-39
-45
n51 2yrs
J Hum Hypertens 1999 13 47-53
16
Ramipril in Diabetic Nephropathy (normotensives)
Microalbuminuria in 24h urine
mg/l
140
120
100
80
Week 0
Week 12
60
40
20
0
Ramipril 1.25 mg (n16)
Placebo (n12)
J Med Assoc Thai 1998 81 671-680
17
Ramipril Efficacy In Nephropathy (REIN) Study
- To test effectiveness of ramipril in limiting
progression of renal disease in nondiabetic
nephropathies - Patients were hypertensive or normotensive
- Pts divided into two groups based on baseline
urinary protein excretion rate - - Stratum 1 1-2.9 g/24h
- - Stratum 2 gt 3 g/24h
- Patients received either ramipril or placebo plus
conventional antihypertensives (except ACEI and
angiotensin II antagonists) to reach diastolic
BPlt90 mmHg - Ramipril dose initiated at 1.25 mg, titrated to
2.5 or 5 mg - Progression of renal disease determined by rate
of decline in GFR
18
REIN Study Results Stratum 2
Rate of decline in GFR and percentage risk of
progression of nephropathy (combined
endpointdoubling of baseline serum creatinine or
endstage renal failure) according to baseline
urinary protein excretion
Kidney failure
GFR decine
t
70
h
n
t
i
1.6
n
o
o
p
Ramipril (n78)
d
m
60
n
r
e
1.4
Placebo (n88)
e
p
d
e
)
n
n
50
i
i
1.2
b
m
m
/
L
o
m
c
40
1.0
(
g
e
n
i
n
h
i
l
0.8
c
c
30
a
e
e
d
r
R
s
0.6
t
F
20
n
G
e
i
t
f
a
0.4
o
p
e
10
f
t
o
a
r
0.2
n
n87
n48
a
0
e
n61
n36
M
0
4.5 to 7.0
gt
7.0
3.0 to 4.5
gt
4.5 to 7.0
7.0
3.0 to 4.5
16 months
Lancet 1997 349 1857-1863
19
REIN Study Results Stratum 1
n186
End-stage renal failure decreased by 56
Am J Health Syst Pharm 2000 57(Suppl 1) S12-S18
20
REIN study Implications
- Reduction in BP was similar in both groups
- Direct renoprotective effect, independent of BP
reduction
Lancet 1997 349 1857-1863
21
Ramipril V/s. Enalapril in Hypertension
Systolic BP
Diastolic BP
0
-2
-4
-6
Reduction in BP (mm Hg)
-8
-7.2
-10
-8.9
-12
-12.2
T/P 64 (R) 47 (E)
-14
-14.6
-16
Ramipril 2.5-5 mg
Enalapril 10-20 mg
n30 8 wks
Br. J Clin Pract 1996 50 302-310
22
APRES (Angiotensin-converting enzyme inhibition
Post Revascularisation Study)
Cardiac death, AMI or clinical heart failure ()
(5mg ? to 10mg after 1 month)
1-2 days post PTCA 5-7 days post CABG
JACC 2000 35 881-888
23
APRES study Clinical implications
- Patients with asymptomatic and moderate LV
dysfunction who undergo revascularisation for
chronic stable angina pectoris carry a
significant risk of cardiac death and other
cardiac events - Long-term treatment with ramipril can reduce this
risk - Benefits with ACE inhibitor treatment should be
extended to this patient group
JACC 2000 35 881-888
24
SECURE (Study to Evaluate Carotid Ultrasound
changes in patients treated with Ramipril and
vitamin E) Study
Progression slopes of the mean maximum carotid
IMT (in mm/year) in the different treatment groups
Treatment group Value Placebo 0.0217 Ram
ipril 10 mg 0.0137
N732
p0.033 vs placebo
Relative reduction in mean maximum IMT was 37
for ramipril 10 mg vs placebo
Circulation 2001 103 919-925
25
SECURE study Mechanisms for the benefit
- Inhibition of tissue and circulating angiotensin
II - Potentiation of bradykinin
- Decreased proliferation and migration of SMCs
- Decreased accumulation and activation of
inflammatory cells - Decreased oxidative stress
- Increased nitric oxide formation, improved
endothelial function
Circulation 2001 103 919-925
26
FDA-Approved Indications for ACE Inhibitors
- Left Prevention of Myocardial Congestive Ventri
cular Infarction, Stroke, andHypertension Heart
Failure Dysfunction Cardiovascular Death - All ACE Captopril Captopril RamiprilInhibitors
Enalapril Enalapril - Fosinopril
Lisinopril - Lisinopril
Ramipril - Quinapril
- Ramipril
- Am J Health Syst Pharm 2000 Suppl 1 S27.