Why Study Continental Aquatic Systems - PowerPoint PPT Presentation
1 / 156
Title: Why Study Continental Aquatic Systems
1
(No Transcript)
2
Types of Aquatic Organisms
- The species concept
- Major taxonomic groups
- Classification of organisms by functional
significance - Organisms found in freshwater systems
3
The Species Concept
- How do you define species?
- How do taxonomists usually define species?
4
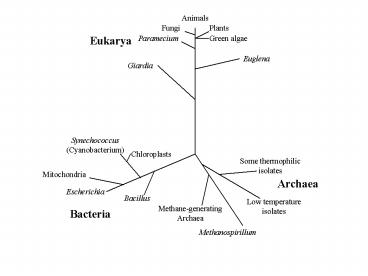
Major Taxonomic Groups
- Archaea
- Bacteria
- Eukarya
- Using rRNA for taxonomic identification of
organisms
5
rna.ucsc.edu/rnacenter/ribosome_images.html
6
Classification of Organisms by Functional
Significance
- Autotrophic versus heterotrophic
- Chemoautotrophic versus photoautotrophic
- Detritivory versus predation
- Functional feeding groups shredders- shred
leaves grazers- eat algae or plants collectors-
collect particles carnivores- eat other animals
7
Classification by Direct Interaction Type
- Competition -/-
- Mutualism /
- Exploitation /-
- Commensalism /0
- Amensalism -/0
- Neutralism 0/0
8
Classification by Habitat
- Memorize all terms on Table 7.3
9
(No Transcript)
10
Microbes and Plants
- Viruses
- Archaea
- Bacteria
- Protoctista
- Fungi
- Plantae
11
Viruses
- All organisms have viruses
- RNA or DNA, capsid (covering) also important
- Prions little understood
12
Virus-Like Particles Common in Lakes
13
(No Transcript)
14
Archaea
- As different from bacteria as eukarya
- Morphologically similar to bacteria
- Originally thought to be mainly extremophiles
(hyperthermic, halophilic, anaerobic) - Now known to occur in all habitats
- Essential in nutrient cycling
15
Bacteria
- Most important organisms in nutrient cycling on
earth - Can only culture lt 1 of all species
- Most species only have few morphologies
- Most identification based on metabolic or
chemical characteristics - Cyanobacteria are included in this group
16
Bacterial Morphologies
10 µm
17
Cyanobacteria
- Produce O2, photosynthetic
- Fix nitrogen with heterocysts
- Float by gas vesicles
- Produce objectionable odors, tastes, and toxins
- Unique light-harvesting pigments
18
Cyanobacterial Morphologies
19
Cyanobacterial Toxins
- Produce hepatotoxins and neurotoxins
- Neurotoxins are highly toxic
- Hepatotoxins damage liver. Low, chronic exposures
may cause liver cancer - Toxins can be bioconcentrated by some organisms,
and influence many different types of animals - Toxins evolved as protection against zooplankton
grazing - Other algae make toxins, red-tide, Prymnesium
parvum responsible for a massive fish kill in
summer 2002 in NE Colorado. Coming to a
reservoir near you soon! Also important in fish
culture ponds
20
Google algal blooms fish kill.any summer
- Second fish kill found--nearly 4 million reported
in Pamlico River - August 6, 2008 - 856PM
- Sun Journal
- Nearly 4 million fish were found dead Wednesday
in the Pamlico River just east of Washington. - It was the second large fish kill reported this
week in Eastern North Carolina rivers. On
Tuesday, an estimated 750,000 dead fish were
found in the Neuse River between Otter Creek and
Carolina Pines.
21
Protoctista
- Eukaryotic Algae
- Protozoa
22
Eukaryotic Algae
- Chrysophyceae- flagellated, and ingest particles
- Bacillariophyceae- diatoms, have silicon frustule
that is useful in paleolimnology, abundant in
many types of freshwaters - Dinophyceae- dinoflagellates, flagellates, some
toxic and cause fish kills - Euglenophyceae- Euglena
- Chlorophyceae and Charophyceae- green algae
23
Protozoa
- Important consumers of bacteria
- Mastigophora- flagellates
- Phytomastigophora- green
- Zomastigophora- colorless
- Sarcodina- amoeboid protozoa
- Cilipophora- ciliates
24
Protozoa
25
Fungi
- Aquatic Fungi
- saprophytic
- some predatory
- Aquatic Lichens
- symbiosis between fungi and alga
- can be very important in some wetlands
26
Plantae
- Nonvascular plants
- Vascular plants
- Large algae and plants called macrophytes, often
classified by growth habit
27
Growth Habit of Macrophytes
28
Non-Vascular Plants
- Bryophytes- mosses and liverworts
- Sphagnum globally important in carbon deposition
in peat bogs - Some aquatic mosses can be found very deep in
oligotrophic lakes - Some streams can be dominated by bryophytes
29
Vascular Plants
- Dominant producers in many wetlands, shallow
lakes and ponds - Many wetlands classified based on the vegetation
that they contain - A wide variety of forms
30
Diversity of Aquatic Plants- Emergent
31
Diversity of Aquatic Plants-Submerged and Floating
32
(No Transcript)
33
Multicellular Freshwater Animals, Invertebrates
- Porifera- sponges
- Cnidaria- include hydra
- Platyhelminthes- include planarians (Turbellaria)
and some important parasites - Gastrotricha- can be abundant, benthic
- Rotifera- rotifers some sexual, others asexual
- Nematoda- important predators and bactivores
- Mollusca- Gastropoda (snails and limpets) and
Bivalva (clams and mussels) - Annelida- segmented worms
- Bryozoa- sessile ciliated invertebrates
- Arthropoda- includes insects, Crustacea, etc.
34
Invasion by Zebra Mussels
- Entered Great Lakes in 1986 from European ships
dumping bilge water - Can produce 1,000,000 eggs each reproductive
cycle, giving rise to easily transported veliger
larvae - Attach to solid substrates and rapidly cover
other organisms - Filter feed and remove substantial amount of
materials from water, improve water clarity and
outcompete other filterers - Will spread through United States eventually,
just made it into Missouri River - Recently entered Kansas waters Cheney, Marion,
Afton reservoirs
35
Zebra Mussel Expansion
2008
36
(No Transcript)
37
Arthropoda
- Class Arichnida- spiders and mites
- Subphylum Insecta
- Collembola
- Ephemeroptera- mayflies
- Odonata- dragonflies
- Plecoptera- stoneflies
- Tricoptera- caddisflies
- Megaloptera and Neuroptera- fissflies,
alderflies, and spongillaflies - Hemitera- true bugs
- Lepidoptera- aquatic caterpillars
- Coleoptera- water beetles
- Diptera- flies and midges
38
Arthropoda, continued
- Suphylum Crustacea
- Ostracoda- seed shrimp
- Copepoda- copepods
- Branchiopoda
- Cladocera- water fleas (Daphnia)
- fairy, tadpole, brine, and clam shrimps (e.g.
Artemia) - Decapoda- crayfish, crabs, shrimps
- Isopoda- isopods
- Amphipoda- scuds and sideswimmers
39
Phylum Chordata, Subphylum Vertebrata
- Fishes
- Tetrapods
- Amphibians
- Urodela or Caudata- salamanders
- Anura- frogs
- Birds, Reptiles, Mammals
40
(No Transcript)
41
Biodiversity of Freshwaters
- Measures of diversity
- Temporal and spatial factors influencing
evolution of freshwater organisms - Short-term factors influencing local distribution
of species - Invasions of nonnative species
- Extinction
- What is the value of freshwater species diversity?
42
Measures of Diversity
- Species richness- the number of species
- Eveness- how well represented each species is
- Shannon-Weiner diversity measure includes both
richness and evenness. - ?- within habitat, and ?- between habitat
diversity
43
? and ? Diversity
B
A
C
D
F
E
G
H
44
Temporal and Spatial Factors Influencing
Evolution of Freshwater Organisms
- Time- ancient lakes and watersheds have many
unique species and high diversity - Lake Baikal has 377 endemic crustacea, 86
Turbellaria, 98 mollusks, 29 fish, and a
freshwater seal - Geographic isolation
- unique groundwater fauna related to isolation
- dispersal ability linked to degree of isolation
- temporary pools can have many endemic species
45
(No Transcript)
46
Short-Term Factors Influencing Local
Distribution of Species
- Colonization
- Habitat type
- Disturbance
- Productivity
- Species interactions
- Species introductions
47
Habitat as Template for Evolution-Habitats from a
Small Lake
48
Species Area Relationships
- S Caz
- Holds from diatoms to fish
49
Invasions of Nonnative Species
- Most permanent form of pollution
- Conceptual model of why species invade
successfully - most invaders fail to establish
- most successful invaders have no significant
effects - all aquatic systems can be invaded
- major community effects occur most often in
low-diversity systems - top predators are more likely to have strong
effects - species must have appropriate physiological and
morphological adaptations to invade successfully - invaders are more likely to become established in
disturbed systems - environmental variability can play a role in
establishment - very stable systems may be vulnerable to invasion
- the greater the number of invaders and the number
of invasions, the greater the probability of
successful invasions - species that have a history of prior invasions
are likely to invade successfully again
50
Invaders of the Great Lakes
51
Extinction
- Extinction rates are about 1 million times
greater than naturally - Aquatic systems are very vulnerable
- Lake Victoria- 300 species evolved in last 12,000
years, 200 extinct in last decade from
introduction of Nile Perch and pollution - In U.S. there are 73 fish, 69 bivalves, 28
snails, 17 amphibians, and 20 crustaceans listed
as threatened or endangered - Over half of the freshwater unionid mussel
species are endangered - Extinction is forever
52
What is the Value of Freshwater Species Diversity?
- What do you think?
53
(No Transcript)
54
Aquatic Chemistry Controlling Nutrient Cycling
Redox and O2
- Chemicals in freshwaters
- Redox potential, potential energy, and chemical
transformations - Oxygen- forms and transformations
- Photosynthesis
- Distribution of dissolved oxygen in the
environment
55
Chemicals in Freshwaters
- Dissolved versus particulate
- Colloidal versus gravitoidal
- Total dissolved solids
- Salinity
- Conductivity
- pH
- Turbidity
- Alkalinity
56
Spatial Scale and Chemicals in Water
57
Correct Version of Fig. 11.3
58
Frequency Distribution of Dissolved Chemicals
59
Redox Potential, Potential Energy, and Chemical
Transformations
- Redox is a measure of free electron availability
- Chemicals have potential energy if they are at a
different redox state than the solution that they
are in
60
Potential Energy and Redox
61
Redox of Biologically Important Molecules
62
Iron and Redox
63
Oxygen- Forms and Transformations
- 21 of atmosphere is O2
- Aerobic/anaerobic- oxic/anoxic
- Oxygen drives redox
- Saturation concentration of dissolved O2 depends
on atmospheric pressure and temperature - Photosynthesis produces O2 , respiration consumes
it. Memorize the equations in the book
64
Photosynthesis
- Net photosynthetic rate gross photosynthetic
rate - respiration - Factors that influence photosynthesis include
light, temperature, water velocity, and chemicals
(e.g. herbicides) - Relationship between photosynthesis and light
referred to as P-I relationship
65
P-I Relationship
66
Photosynthesis and Temperature
67
Photosynthesis and Water Velocity
68
(No Transcript)
69
Spatial Variation in O2
70
Temporal Variation in O2
71
(No Transcript)
72
Carbon
- Forms of carbon
- Transformations of carbon
- A general introduction to nutrient cycling and
the carbon cycle
73
Forms of Carbon
- Inorganic carbon-bicarbonate equilibrium
- carbon dioxide CO2
- carbonic acid H2CO3
- bicarbonate HCO3-
- carbonate CO32-
- CO2 H2O? H2CO3 ?HCO3- H ?CO32- 2H
- Organic carbon
74
pH and Bicarbonate Equilibrium
75
Lake Nyos Disaster
- 1700 people and many livestock died near Lake
Nyos in Cameroon in 1986 - A survivor reported a 25m high water surge and
odor of rotten eggs - Caused by catastrophic release of supersaturated
CO2 from the hypolimnion - CO2 probably came from volcanic activity
- Landslide or cool weather released the gas
- Building up again, using pipes to release
pressurized water
76
Photo Bernard Canet, March 1995
http//perso.wanadoo.fr/mhalb/nyos/index.htm
77
Organic Carbon
- Dissolved versus particulate (DOC vs POC)
- CPOM and FPOM
- Biochemical Oxygen Demand (BOD)
- Tannins, lignins, cellulose
- Humic materials
- humic acids (soluble in alkaline precip in acid)
- fulvic acids (soluble in acid)
- humins (not extractable by acid or base)
78
Transformations of Carbon
- Oxidation of organic carbon with inorganic
electron acceptors other than O2 - Fermentation
- Methanotrophy
- Methanogenesis
- Autotrophy (photoautotrophy includes oxygenic and
anoxygenic photosynthesis) - Respiration
79
Aerobic Carbon Breakdown
80
Anaerobic Carbon Transformations
81
Methanotrophy and Methanogenesis
- Methanotrophs are aerobic organisms that eat
methane and carbon monoxide. Very important in
global carbon cycle - Methanogens make methane from CO2 and H2 at very
low redox. Also can use acetate. Important in
global methane cycle
82
Wetlands and the Global Methane Budget
83
A General Introduction to Nutrient Cycling and
the Carbon Cycle
- A general method for diagramming nutrient cycles
- The carbon cycle
84
(No Transcript)
85
Nitrogen, Sulfur, Phosphorus, and Other Nutrients
- Nitrogen
- Sulfur
- Phosphorus
- Silicon, Iron, and other trace nutrient cycles
- Gradients of redox and nutrient cycles and
interactions among the cycles
86
Nitrogen
- Nitrogen Forms
- N2 gas, N2O nitrous oxide, NH4 ammonium, NO2-
nitrite, NO3- nitrate - Nitrogen Fluxes
- uptake, remineralization, denitrification,
nitrification, dissimilatory nitrate reduction,
nitrogen fixation - Nitrogen Cycle
87
(No Transcript)
88
Nitrogen Fixation
- N2 gas to ammonium, very expensive energetically
- Only bacteria known to fix nitrogen
- Nitrogenase sensitive to O2, and a variety of
adaptations protect it - Lightning also fixes N2 to NO3- in the atmosphere
- Nitrogen-fixing cyanobacteria can be very
important in lake N cycles
89
Cyanobacterial Heterocysts
90
N Cycling
- Nitrification- oxidation of ammonium to nitrite
(Azotobacter) and nitrite to nitrate
(Nitrobacter) - Denitrification- using NO3- as an electron
acceptor for oxidation of carbon, yields N2O and
N2. Drives N loss from environment. Under very
low redox, can go to ammonium - Remineralization (ammonification)
91
Nitrate Contamination
- Nitrate not allowed in drinking water in U.S.
over 10 mg/L - Can lead to methhemoglobinemia, blue baby
syndrome - Can be converted to carcinogenic nitrosamines in
the stomach
92
N Distribution in a Lake
93
N Distribution in a Stream
94
Be Able to Draw the N Cycle from Scratch
95
Sulfur
- Forms (only some listed)
- S2-, sulfide S0, elemental sulfur S2O32-,
thiosulfate SO42-, organic S, dimethyl sulfide - Sulfur Transformations
- Abiotic oxidization (spontaneous conversion to
sulfate, slow) - Biotic oxidation (chemoautotrophic bacteria)
96
More Sulfur Transformation
- Dissimilatory sulfur reduction
- sulfate and successively reduced sulfur compounds
used as electron acceptors for carbon oxidation - Disproportionation
- two sulfurs in thiosulfate, one used to oxidize
the other and energy is produced - S2O32-? S2- SO42- H energy
- Precipitation of metal sulfides
- Anoxygenic photosynthesis- uses sulfide as an
electron donor for photosynthesis and produces
sulfate
97
Be Able to Draw Complete Sulfur Cycle From Scratch
98
Phosphorus
- Forms
- organic P phosphate, PO43-
- Transformations
- uptake
- remineralization (phosphatases)
- precipitation with oxidized iron
99
Silicon, Iron, and Other Trace Nutrient Cycles
- Silicon
- key element in diatom frustules
- can become limiting in lakes
- Iron
- ferric, Fe3, oxidized ferrous, Fe2 reduced
- iron oxidation by microorganisms important
chemoautotrophic pathway, but also will happen
abiotically, so must occur at oxic/anoxic
interface - oxidized iron precipitates with phosphate, but
dissociates again in anoxic conditions - Chelators can keep iron in oxic solutions
100
Annual Cycle of Silicon in a Lake
101
Sulfur-Iron Dynamics and Wetland Eutrophication
- Phosphorus pollution in peaty lowlands of
Netherlands encouraged unwanted algal blooms and
hurt macrophyte populations - Put low P, high sulfate river water in
- Sulfate was reduced to sulfide, which
precipitated iron and poisoned roots of
macrophytes - Iron was not present to bind to phosphates and
phosphate concentrations actually increased
102
Gradients of Redox and Nutrient Cycles and
Interactions Among the Cycles
103
(No Transcript)
104
Effects of Toxic Chemicals and Other Pollutants
on Aquatic Ecosystems
- Basic toxicology
- Bioassessment
- Acid precipitation
- Metals and other inorganic pollutants
- Organic pollutants
- Suspended solids
- Thermal pollution
105
Basic Toxicology
- Acute vs. chronic exposure
- lethal/ sublethal/ cumulative
- LD50, EC50
- Additive versus multiplicative effects
- Effects of other factors on toxins
- Bioconcentration- moving into organisms
- Bioaccumulation- accumulation from food (lipid
solubility) - Biomagnification- increases as you move up the
food web
106
Bioassessment
- Use native assemblages to indicate chronic
toxicity - Useful because long term effects are difficult to
document and acute episodes may be hard to sample - EPT index is one common tool for assessment
- Index of Biotic Integrity (Karr) assesses
habitats and fish species
107
Relationship between Invertebrate Diversity and
some Environmental Parameters
108
Acid Precipitation
- Sources and geography of acid precipitation
- Biological effects of acidification
109
Sources and Distribution of the Problem in the
United States
110
(No Transcript)
111
(No Transcript)
112
(No Transcript)
113
(No Transcript)
114
(No Transcript)
115
(No Transcript)
116
(No Transcript)
117
(No Transcript)
118
(No Transcript)
119
(No Transcript)
120
(No Transcript)
121
(No Transcript)
122
(No Transcript)
123
(No Transcript)
124
(No Transcript)
125
(No Transcript)
126
(No Transcript)
127
(No Transcript)
128
(No Transcript)
129
(No Transcript)
130
(No Transcript)
131
(No Transcript)
132
(No Transcript)
133
(No Transcript)
134
(No Transcript)
135
(No Transcript)
136
(No Transcript)
137
(No Transcript)
138
(No Transcript)
139
(No Transcript)
140
(No Transcript)
141
(No Transcript)
142
(No Transcript)
143
(No Transcript)
144
(No Transcript)
145
(No Transcript)
146
Influences of Decreased pH on Aquatic Organisms
147
Influence of Decreased pH on Bacterial Activity
148
Paleolimnology to Show Trends in Acidification
149
Increased Aluminum with Lower pH
150
Aluminum and Fish
151
Metals and Other Inorganic Pollutants
- Lead toxicity and waterfowl
- Mercury contamination of fish. Atmospheric
deposition followed by methylation - Selenium concentrates in some areas as water
evaporates. A cofactor at low concentrations but
toxic at high - Arsenic in groundwater
152
Organic Pollutants
- More than 10,000 created and used by humans,
several hundred new each year - Petroleum products. Urban runoff. A 20hp 2
stroke engine can make 11,000 m3 of water
undrinkable in one hour - Chlorinated hydrocarbons concentrate in sediments
- Atrazine common in agricultural areas. Poisons
algae and macrophytes, may poison frogs
153
Endocrine Disrupting Compounds
- Ecoestrogens mimic estrogen, many compounds
including DDT - Active at minute concentrations
- May cause disruption in sex determination in
animals - Can be bioconcentrated and passed to offspring
- Combinations of organic chemicals may interact
with estrogen acceptors
154
Bioremediation
- Using organisms to mediate pollution
- Usually bacteria
- Bacteria rapidly evolve the ability to withstand
and utilize organic compounds (e.g. antibiotic
resistance) - Plasmids transfer genetic information readily
- In situ bioremediation is often the cheapest way
to clean up organic pollution, particularly in
groundwater
155
Suspended Solids
- Can smother invertebrates
- Also serve as food source for filtering
invertebrates - Largest mass of any pollutant
- Can fill gravel and interfere with fish
reproduction - Can increase light extinction rates, and lower
phytoplankton production
156
Thermal Pollution
- Power plants need cooling water
- Increased temperatures can have adverse
environmental impacts, including - extend range of exotic invaders
- interfering with reproductive cycles and timing
- death of heat intolerant species
- This research now relevant to global warming