I'Atomic Masses - PowerPoint PPT Presentation
1 / 9
Title:
I'Atomic Masses
Description:
A mass spectrometer can compare other elements or isotopes to 12C ... 3.2 What is the mass of 6 Americium atoms? Calculating moles, mass, and atoms. 1. Sample Ex. ... – PowerPoint PPT presentation
Number of Views:64
Avg rating:3.0/5.0
Title: I'Atomic Masses
1
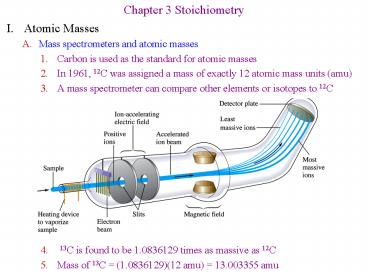
Chapter 3 Stoichiometry
- I. Atomic Masses
- Mass spectrometers and atomic masses
- Carbon is used as the standard for atomic masses
- In 1961, 12C was assigned a mass of exactly 12
atomic mass units (amu) - A mass spectrometer can compare other elements or
isotopes to 12C - 13C is found to be 1.0836129 times as massive as
12C - Mass of 13C (1.0836129)(12 amu) 13.003355 amu
2
- Average Atomic Masses
- Why isnt the mass of Carbon listed at exactly 12
on the periodic table? - Natural carbon 12C(98.89), 13C(1.11), and 14C
(negligible) - Avg. Mass (0.9889)(12 amu) (0.0111)(13.0034
amu) 12.01 amu - No individual atoms have this mass
- On average, all natural carbon atoms have this
mass - 12.01 amu is the correct value to use for
calculations involving carbon - Sample Ex. 3.1 What is avg. mass of Cu?
- 69.09 63Cu (62.93 amu) and 30.91 65Cu (64.93
amu) - Calculate the Mass (in amu) of 75 atoms of Al
- a. Determine the mass of 1 Al atom 1 atom of Al
26.98 amu - b. Use the relationship as a conversion factor
3
- II. The Mole
- We use a package for atoms and molecules called a
mole - A mole
- a. the number of Carbon atoms in 12 g of 12C
- 6.022 x 1023 units Avogadros Number
- The amount of an element equal to its atomic mass
- 1 mole of natural C atoms weighs 12.01 g and has
6.02 x 1023 atoms - 1 mole of He atoms weighs 4.003 g and has 6.02 x
1023 atoms - 1 mole of Al atoms weighs 26.98 g and has 6.02 x
1023 atoms - Sample Ex. 3.2 What is the mass of 6 Americium
atoms? - Calculating moles, mass, and atoms
- 1. Sample Ex. 3.3 atom / moles in 10g Al
Cu
I2
Hg
Al
Fe
S
4
- 2. Sample Ex. 3.4 5.68 mg Si ? atoms Si
- Molar Mass
- 1. The molar mass is the mass in grams of one
mole of a compound - 2. The relative weights of molecules can be
calculated from atomic masses - a. Water H2O 2(1.008 g/mol) 16.00 g/mol
18.02 g/mol - b. 1 mole of H2O will weigh 18.02 g, therefore
the molar mass of H2O is 18.02 g - 1 mole of H2O will contain 16.00 g of oxygen and
2.02 g of hydrogen - Calculations with molar mass
- a. Sample Ex. 3.6 Juglone C10H6O3 Find Molar
mass and moles in 0.0156 g
5
- Sample Ex. 3.8 How many molecules of isopentyl
acetate (C7H14O2) and how many carbon atoms are
released in one bee sting (1 mg)? - D. Mass Percent
- Composition of a substance can be given multiple
ways - Molecular formula gives ratio of the number of
each element - The Mass Percent gives the composition by mass of
each element - Example Ethanol, C2H6O has a molar mass of 46.07
g
6
- Sample Ex. 3.10 Determine the Mass Percent of
C14H20N2SO4. - III. Determining the Formula of a Compound
- Suppose we make a new compound composed of C,H,
and N - We probably know what it was we were trying to
make - That doesnt mean we succeeded
- We must have evidence that the compound we made
is what we wanted - Lets turn a weighed amount of the compound into
CO2, and H2O
7
- B. Analyzing the Results
- 0.1156 g of our new compound gives 0.1638 g CO2
and 0.1676 g H2O - (1C)(12.01g/C) (2 O)(16.00g/O) 44.01 g total
for CO2 - (2H)(1.008g/H) (1 O)(16.00g/O) 18.02 g total
for H2O - Next, we find out how much carbon and hydrogen
was in our sample - Then, we determine the Mass Percents for our new
compound - Finally, we need to determine the molecular
formula of our compound - The easiest way to do this is to work with a
theoretical 100.00g - We can then change the percent masses to grams of
each element - 38.67 g Carbon, 16.22 g Hydrogen, 45.11 g Nitrogen
8
- Molecular formulas compare moles, not grams
- Write the formula with these numbers of moles,
then divide by the smallest number to get these
subscripts as whole numbers - This is called the Empirical Formula smallest
whole number ratio of elements in the formula - Molecular Formula is the actual number of each
element - Could be C2H10N2 or C3H15N3 etc
- (C1H5N1)n all possible molecular formulas for
this empirical formula - We can use a Mass Spectrometer on our compound to
find its molecular mass is 31.06 g/mol. In this
case CH5N is correct molecular formula. - C. Sample Ex. 3.113.13 give more practice
9
- Hints for determining empirical and molecular
formulas - Convert mass to grams of the element in 100
total grams of sample - Determine the Empirical Formula of an unknown if
its Percent Composition is 47 Carbon, 47 Oxygen
and 6.0 Hydrogen - Change these masses to moles by using the atomic
mass of each element - Divide each number of moles by the smallest to
get a small ratio - Round off to a whole if molar s are close to
that whole number - Multiply the whole ratio by a factor to get all
numbers to whole numbers - Multiply empirical formula by factor needed to
give the correct molar mass - Molar Mass of unknown 204.2 g/mol but C4H6O3
102.09 g/mol - Correct Molecular Formula C4H6O3 x 2 C8H12O6