QUANTITATIVE ELECTROLYSIS (Unit 3 PPA 2) - PowerPoint PPT Presentation
QUANTITATIVE ELECTROLYSIS (Unit 3 PPA 2)
... to produce one mole of hydrogen by electrolysis of dilute sulphuric acid (1) What measurements would be taken in this experiment? (1) The current in amps. ... – PowerPoint PPT presentation
Title: QUANTITATIVE ELECTROLYSIS (Unit 3 PPA 2)
1
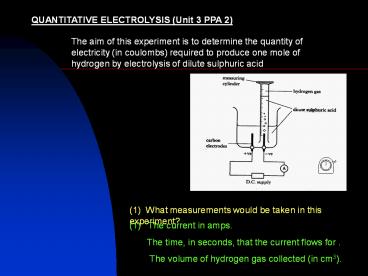
QUANTITATIVE ELECTROLYSIS (Unit 3 PPA 2)
The aim of this experiment is to determine the
quantity of electricity (in coulombs) required to
produce one mole of hydrogen by electrolysis of
dilute sulphuric acid
(1) What measurements would be taken in this
experiment?
(1) The current in amps. The time, in
seconds, that the current flows for . The
volume of hydrogen gas collected (in cm3).
2
(2) In an experiment it was found that a current
of 0.4 amps flowing for 200 seconds
produced 9.9 cm3 of hydrogen gas. Given that
the molar volume of a gas under the experimental
conditions is 23.8 litres, calculate the
number of coulombs needed to produce 1 mole
of hydrogen.
(2) Q I x t 0.4 x 200
80 C 9.9 cm3 of hydrogen was produced
by 80 Coulombs So 1 mole, 23800 cm3, of hydrogen
is produced by 80 x 23800/9.9 192 323 C
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics, the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.