Lucky 13 and Equilibrium - PowerPoint PPT Presentation
1 / 14
Title:
Lucky 13 and Equilibrium
Description:
... will shift the equilibrium to the _ (removal of heat has the opposite effect) ... b. 1.0 mole pure HOCl is placed in a 2.0 L flask. ... – PowerPoint PPT presentation
Number of Views:135
Avg rating:3.0/5.0
Title: Lucky 13 and Equilibrium
1
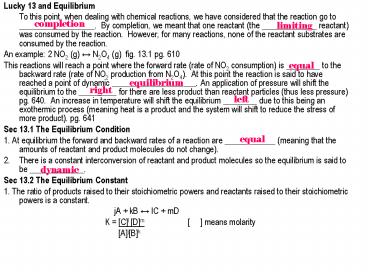
- Lucky 13 and Equilibrium
- To this point, when dealing with chemical
reactions, we have considered that the reaction
go to __________________. By completion, we
meant that one reactant (the _____________
reactant) was consumed by the reaction. However,
for many reactions, none of the reactant
substrates are consumed by the reaction. - An example 2 NO2 (g) ? N2O4 (g) fig. 13.1 pg.
610 - This reactions will reach a point where the
forward rate (rate of NO2 consumption) is
________ to the backward rate (rate of NO2
production from N2O4). At this point the
reaction is said to have reached a point of
dynamic ____________________. An application of
pressure will shift the equilibrium to the
_________ for there are less product than
reactant particles (thus less pressure) pg. 640.
An increase in temperature will shift the
equilibrium ________ due to this being an
exothermic process (meaning heat is a product and
the system will shift to reduce the stress of
more product). pg. 641 - Sec 13.1 The Equilibrium Condition
- 1. At equilibrium the forward and backward rates
of a reaction are ____________ (meaning that the
amounts of reactant and product molecules do not
change). - There is a constant interconversion of reactant
and product molecules so the equilibrium is said
to be _____________. - Sec 13.2 The Equilibrium Constant
- 1. The ratio of products raised to their
stoichiometric powers and reactants raised to
their stoichiometric powers is a constant. - jA kB ? lC mD
- K
Cl Dm means molarity - AjBk
completion
limiting
equal
equilibrium
right
left
equal
dynamic
2
initial
- See table 13.1 pg. 616 to see that K is constant
at a given temperature regardless of the
___________ set of concentrations of reactants or
products (at equilibrium, the concentrations are
called the equilibrium positions). There are
an _____ number of equilibrium positions but only
___ value of equilibrium constant for a given
reaction at a given temperature (as seen earlier
temperature can shift the equilibrium). - 2. The larger the equilibrium constant, the more
the reaction will tend toward completion (but the
size of K says nothing of the ______ of the
reaction which is determined by the size of the
activation energy barrier). - Haber Process N2 (g) 3 H2 (g) ? 2NH3 (g) See
Summary Table pg. 615 - Sample Exercise 13.1-13.3
8
1
rate
13.2b.
K N2H23 NH32
13.2c. K NH3
N21/2H23/2
13.2a. K NH32
N2H23
13.1 K NO24H2O6
NH34O27
K 1 3.795.104
K 0.0312
0.850.00313
K (3795)1/2
K 190
K 3.8.104
K 2.6.10-5
Sec 13.3 Equilibrium Expressions Involving
Pressure 1. Concentration is related to pressure
through PV nRT or n / V P / RT or C
P / RT (could that be an ice cream P/RT?) so P
CRT. 2. An equilibrium expression can be written
as pressures. Kp PNH32 Kc NH32 Kp NH3
2 (RT)2 Kp Kc (RT)-2 PN2 PH23
N2H23
N2(RT)H23(RT)3 (see pg. 619) Kp
KC (RT)?n ?n ?n products - ?n reactants
3
Sample Exercise 13.4 pg. 618-620
Sample Exercise 13.5 pg. 618-620
Kp PNOCl2 PNO2 PH2
Kp KC( RT)?n
Kc Kp /( RT)?n
Kp (1.2 atm) 2 (0.050 atm)2(0.30 atm)
KC 1920 atm-1 / (0.08206 Latm/molK(298K))2-3
Kp 1.9.103 atm-1
KC 1920 / (0.08206(298))-1
KC 4.7.104
- Sec 13.4 Heterogeneous Equilibria
- 1. So far we have examined equilibria where all
species are gases (one phase). A homogeneous
equilibrium consists of ________ phase (such as
gases). Many equilibria contain more than one
phase (any combination of solids, liquids or
gases) and are called _______________________
equilibria. - 2. Experimental results have shown the
equilibrium constants do not depend upon
_________ of solids or liquids (as their
concentrations of pure substances cannot change).
- 3. Since the concentrations do not change, pure
solids and liquids are _______________ from the
equilibrium constant expressions. - Sample Exercise 13.6 pg. 621-622
one
heterogeneous
amount
excluded
Homework pg. (dont need to write 9, 10, 11
15) write 19a,b 20 c,d 21 25 29 31 42 (use
the chart technique
4
- Sec 13.5 Applications of the Equilibrium
Constant - Knowledge of the equilibrium constant indicated
the tendency for a reaction to occur. The larger
the equilibrium constant the ______ likely a
reaction is to occur (as discussed yesterday K
gives no indication of rate of reaction). - Reaction Quotient
- 1. If the reactants of a reaction are mixed with
no products, the system will shift to the
_________ to produce products until the
equilibrium condition is attained. If only
products are mixed then the system will shift
________ to produce reactants until an
equilibrium is attained. How do we see which way
some set of initial concentrations of reactants
and products will shift? Answer Reaction
quotient. - 2. The reaction quotient (Q) is calculated in the
_________ same fashion as the equilibrium
constant (K), the only difference is the Q is
calculated at the time the reactants and products
are originally mixed (before the shift). - 3. If Q gt K, then there is too large of a ratio
of product to reactant and the system will shift
_________. - If Q lt K, then there is too large of a ratio
of reactant to product and the system will shift
_________. - If Q K then the system is in a state of
___________________ (no shift). - Dont worry to show the units on K, Kp or Q.
- See example 13.7 pg. 625
more
right
left
exact
left
right
equilibrium
5
- Exercise 13.7
- Q NH32
- N2 H23
- Q 1.0.10-32
- 1.0.10-52.0.10-33
- Q 1.3 .107
- Since Q gt K system will shift left to attain
equilibrium - b. Q 2.00.10-42
- 1.50.10-53.54.10-13
- Q 6.0 .10-2
- Since Q K system is at equilibrium (no shift)
- c. Q 1.0.10-42
- 5.0 1.0.10-23
- Q 2.0 .10-3
- Since Q lt K system will shift right to attain
equilibrium
6
- Equilibrium Problems
- Sample Exercises 13.8 13.11 pg. 626-635
- When equilibrium constants are small the amount
removed or added to some initial concentration
can be neglected (use the 5 rule).
7
- Sample Exercise 13.8
- N2O4 (g) ? 2 NO2(g) Kp 0.133
- 2.71 atm ?
- KP PNO22
- P N2 O4
- P NO2 (Kp P N2O4)1/2
- P NO2 (0.133(2.71 atm))1/2
- P NO2 0.600 atm
- Sample Exercise 13.9
- PCl5 (g) ? PCl3 (g) Cl2
(g) K ? - Initial 0.00870 mol 0.298 mol 0 mol (no
product so shift right) - - 0.00200 mol
0.00200 mol - final 0.006700 mol 0.300 mol 0.00200
mol - Final concentrations
- 0.006700 mol / 1.00 L 0.300 mol /
1.00 L 0.00200 mol / 1.00 L - K 0.30000.002000
- 0.006700
- K 8.96.10-2
8
- Exercise 13.10
- CO (g) H2O (g) ? CO2(g) H2 (g) K
5.10 - Initial 1.000 mol 1.000 mol
1.000 mol 1.000 mol - mixed in a 1.000 L flask so all initial
molarities are 1.000 M - Q CO2H2
- CO H2O
- Q 1.00 Since Q lt K system will shift right
- CO (g) H2O (g) ?
CO2(g) H2 (g) - Final 1.000 M -x 1.000 M -x
1.000 M x 1.000 M x - K 1.00 x2
- 1.00 x2
- (K)1/2 (1.000 x) / (1.000 x)
- (5.10)1/2 - (5.10)1/2 x 1.000 x
- 1.2583 3.3583 x
- x 1.2583 / 3.3583 0.38618 M
CO H2O (1.000 M 0.38618 M) 0.614 M
CO2 H2 (1.000 M 0.38618 M) 1.386 M
Check K CO2 H2 CO
H2O K 1.38618 2
0.613822
K 5.10
9
- Exercise 13.11
- H2 (g) F2 (g) ? 2 HF (g) K 115
- 3.000 mol 3.000 mol
3.000 mol - For initial concentrations you must divide by the
volume (1.500 L) - initial 2.0000 M 2.0000 M
2.0000 M - Q HF2
- H2 F2
- Q 2.00002
- 2.00002.0000
- Q 1.000
- Since Q lt K system will shift right
- H2 (g) F2 (g) ? 2 HF (g)
- final 2.0000 x 2.0000 x
2.0000 2x
115 (2.0000 2x)2 / ((2.0000 x)(2.0000
x)) 115 (2.0000 2x)2 / (2.0000 x)2 (115)1/2
(2.0000 2x) / (2.000 x) 21.448 10.724 x
2.0000 2x 12.724 x 19.448 x 19.448 /
12.724 x 1.5285
Final 0.472 M 0.472 M 5.51 M
10
- Exercise 13.12
- H2 (g) I2 (g) ?
2 HI (g) Kp 100. - initial 0.01000 atm 0.005000 atm
0.5000 atm - final 0.01000 atm x 0.005000 atm x
0.5000 atm - 2x - 0.04548 atm 0.04048
atm 0.4290 atm - Q (0.5000 atm)2
- (0.01000 atm) (0.005000 atm)
- Q 5000.
- Since Q gt K system will shift left
- 100. (0.5000 -2x )2
- (0.01000 x)(0.005000 x)
- 100. 0.25000 2.0000 x 4x2
- 0.000050000 0.015000 x x2
- 0.0050000 1.5000 x 100.x2 0.25000 2.0000
x 4x2 - 96 x2 3.5000 x 0.2450 0
- x2 0.0364583 x 0.0025521 0 use Quadratic
x (-b /- (b2-4ac)1/2)/2a - x 0.0354775 (lots of /- rule so dont worry
about it here)
11
Homework Day 1 pg. 645-647 s 13 35 37a,b
39 41 43 45 Additional (Hint when reactions
equations are added, equilibrium constants are
multiplied) Determine the equilibrium constant
for the following Na2O (s) ½ O2 (g) ? Na2O2
(s) K ? Na2O (s) ? 2Na(l) ½ O2 (g) K1
2.10-25 NaO (g) ? Na (l) ½ O2 (g) K2
2.10-5 Na2O2 (s) ? 2Na (l) O2 (g) K3
5.10-29 NaO2 (s) ? Na (l) O2 (g) K4
3.10-14 Day 2 pg. 645-650 s 20a,b 30 36
44 47 50 51a (neglect 2x taken from NOCl) 76a
Additional (Hint when reactions equations are
added, equilibrium constants are
multiplied) Determine the equilibrium constant
for the following a. NaO (g) Na2O (s) ? Na2O2
(s) Na (l) K ? (see reactions of Day 1
additional) b. 2 NaO (g) ? Na2O2 (s) K ?
12
- Shifting Principles
- Sec 13.7 Le Chateliers Principle
- 1. When dealing with a particular reaction that
exists in an equilibrium condition, chemists try
to find ways to _________ the equilibrium so as
to obtain as much product as possible. In order
to know how to do this it is important to
understand ____________ which can shift the
equilibrium. - These factors include concentration, pressure and
temperature. - 2. Le Chateliers principle states that if a
stress is imposed on a system in equilibrium, the
position of the equilibrium will _________ to
reduce the stress placed upon the equilibrium.
For 2 of these factors (concentration and
pressure) the position of the equilibrium may be
shifted but the value of __ will still be
constant. - 3. Changing temperature can shift the
equilibrium, but __ is also dependent upon the
temperature. - The Effect of a Change in Concentration
- 1. The effect of concentration has already been
explored through the use of ___. - 2. An increase in concentration of reactant will
shift the equilibrium _______ (if there is more
than one reactant, the other reactant will
decrease in concentration, the products will
increase in concentration). - 3. An increase in concentration of product will
shift the equilibrium ________ (recall addition
of pure solid or liquid will not alter the
equilibrium). - See pg. 637 for an example.
- Sample Exercise 13.13 pg. 638.
shift
factors
shift
K
K
Q
right
left
13
- The Effect of a Change in Pressure
- 3 ways of changing the pressure on a reaction
system - 1. _____ (or remove) gaseous reactant or product
(same effect as we explored above when adding
reactant or product). - 2. Addition of an ________ gas (one that is not
involved in the reaction). No effect on
equilibrium. - 3. Change the volume of the container. When
decreasing volume, equilibrium will shift to the
side of the reaction with the __________ number
of gaseous particles (since the volume of the
container is reduced, the system tries to reduce
its own volume). Increasing volume has the
opposite effect. - Sample Exercise 13.14 pg. 640.
- The Effect of a Change in Temperature
- 1. Altering the temperature will alter ___. By
a knowledge of the enthalpy of a reaction (?H),
we can predict the direction the equilibrium will
shift. - a. When ?H is __ (an exothermic reaction), heat
is a ___________ of the reaction. Therefore,
adding heat will shift the equilibrium to the
_______ (removal of heat has the opposite effect) - b. When ?H is ___ (an endothermic reaction), heat
is a __________. The application of heat will
shift the equilibrium to the __________ (removal
of heat will have the opposite effect). - Sample Exercise 13.15 pg. 642
- Table 13.4 pg. 642
add
inert
least
K
-
product
left
reactant
right
14
Homework pg. 645-649 s 16 (dont have to
write) 57-61 63 64 Additional at 25oC, K
0.090 for the reaction H2O (g) Cl2O (g) ?
2HOCl Determine the concentrations of all species
at equilibrium for each of the following
cases a. 1.0 g H2O and 2.0 g Cl2O are mixed in a
1.0 L flask. b. 1.0 mole pure HOCl is placed in a
2.0 L flask.