Equilibrium Conversion - PowerPoint PPT Presentation
1 / 44
Title:
Equilibrium Conversion
Description:
... coolers 90% conversion can be achieved compared to an equilibrium conversion of ... A cooling coil has been located in equipment storage for use in the ... – PowerPoint PPT presentation
Number of Views:387
Avg rating:3.0/5.0
Title: Equilibrium Conversion
1
Equilibrium Conversion
The highest conversion that can be achieved in
reversible reactions is the equilibrium
conversion. For endothermic reactions, the
equilibrium conversion increases with
temperature up to a maximum of 1.0. For
exothermic reactions, the equilibrium conversion
decreases with increasing temperature.
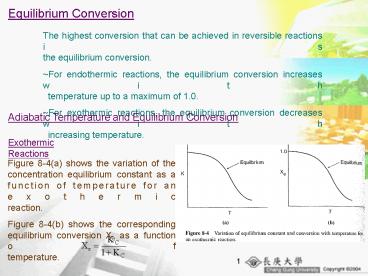
Adiabatic Temperature and Equilibrium Conversion
Exothermic Reactions
Figure 8-4(a) shows the variation of the
concentration equilibrium constant as a function
of temperature for an exothermic
reaction. Figure 8-4(b) shows the corresponding
equilibrium conversion Xe as a function of
temperature.
2
To determine the maximum conversion that can be
achieved in an exothermic reaction carried out
adiabatically, we find the intersection of the
equilibrium conversion as a function of
temperature with temperature-conversion
relationships from the energy balance as shown
in Figure 8-5.
If the entering temperature is increased from T0
to T01, the energy balance line will be shifted
to the right and will be parallel to the original
line, as shown by the dashed line. Note that as
the inlet temperature increases, the adiabatic
equilibrium conversion decreases.
3
Example 8-6
For the elementary solid-catalyzed liquid-phase
reaction make a plot of equilibrium conversion
as a function of temperature. Determine the
adiabatic equilibrium temperature and conversion
when pure A is fed to the reactor at a
temperature of 300 K.
Solution
Equilibrium
Rate Law
Stoichiometry (liquid phase, vv0)
4
Equilibrium Constant
Equilibrium Conversion from Thermodynamics
5
(No Transcript)
6
Reactor Staging with Interstate Cooling of Heating
Higher conversions than those shown in Figure
E8-6.1 can be achieved for adiabatic operation by
connecting reactors in series with interstage
cooling
The conversion-temperature plot for this scheme
is shown in Figure 8-6. We see that with three
interstage coolers 90 conversion can be achieved
compared to an equilibrium conversion of 40 for
no interstage cooling.
7
Endothermic Reactions
The first reaction step (k1) is slow compared to
the second step, and each step is highly
endothermic. The allowable temperature range for
which this reaction can be carried out is quite
narrow Above 530?C undesirable side reactions
occur, and below 430?C the reaction virtually
does not take place.
A typical feed stock might consist of 75
straight chains, 15 naphthas, and 10 aromatics.
Note that the reactors are not all the same size.
Typical sizes are on the order of 10 to 20 m high
and 2 to 5 m in diameter.
Because the reaction is endothermic, equilibrium
conversion increases with increasing temperature.
A typical equilibrium curve and temperature
conversion trajectory for the reactor sequence
are shown in Figure 8-8.
8
Example 8-7
What conversion could be achieved in Example 8-6
if two interstage coolers that had the capacity
to cool the exit stream to 350 K were available?
Also determine the heat duty of each exchanger
for a molar feed rate of A of 40 mol/s. Assume
that 95 of equilibrium conversion is achieved in
each reactor. The feed temperature to the first
reactor is 300 K.
Solution
1. Calculate Exit Temperature
We now cool the gas stream exiting the reactor at
460 K down to 350 K in a heat exchanger.
9
2. Calculate the Heat Load
no reaction in the exchanger
220 kcal/s must be removed to cool the reacting
mixture from 460 K to 350 K for a feed rate of 40
mol/s
3. Calculate the Coolant Flow Rate
10
4. Calculate the Heat Exchange Area
5. Second Reactor
11
Optimum Feed Temperature
Consider an adiabatic reactor of fixed size or
catalyst weight and investigate what happens as
the feed temperature is varied. The reaction is
reversible and exothermic.
At one extreme, using a very high feed
temperature, the specific reaction rate will be
large and the reaction will proceed rapidly, but
the equilibrium conversion will be close to zero.
As a result, very little product will be formed.
At the other extreme of low feed temperatures,
little product will be formed because the
reaction rate is so low. A plot of the
equilibrium conversion and the conversion
calculated from the adiabatic energy balance, is
shown in Figure 8-9. We see that for an entering
temperature of 600 K the adiabatic equilibrium
conversion is 0.15.
The corresponding conversion profiles down the
length of the reactor are shown in Figure 8-10.
The equilibrium conversion also varies along the
length of the reactor as shown by the dashed line
in Figure 8-10. We also see that at the high
entering temperature, the rate is very rapid and
equilibrium is achieved very near the reactor
entrance.
12
We notice that the conversion and temperature
increase very rapidly over a short distance
(i.e., a small amount of catalyst). This sharp
increase is sometime referred to as the point
or temperature at which the reaction ignites.
If the inlet temperature were lowered to 500 K,
the corresponding equilibrium conversion is
increased to 0.38 however, the reaction rate is
slower at this lower temperature so that this
conversion is not achieved until closer to the
end of the reactor. If the entering temperature
were lowered further to 350 K, the corresponding
equilibrium conversion is 0.75, but the rate is
so slow that a conversion of 0.05 is achieved for
the specified catalyst weight in the reactor.
At a very low feed temperature, the specific
reaction rate will be so small that virtually all
of the reactant will pass through the reactor
without reacting. It is apparent that with
conversions close to zero for both high and low
feed temperatures there must be an optimum feed
temperature that maximizes conversion.
As the feed temperature is increased from a very
low value, the specific reaction rate will
increase, as will the conversion. The conversion
will continue to increase with increasing feed
temperature until the equilibrium conversion is
approached in the reaction. Further increases in
feed temperature for this exothermic reaction
will only decrease the conversion due to the
decreasing equilibrium conversion. This optimum
inlet temperature is shown in Figure 8-11.
13
CSTR with Heat Effects
Although the CSTR is well mixed and the
temperature is uniform throughout the reaction
vessel, these conditions do not mean that the
reaction is carried out isothermally. Isothermal
operation occurs when the feed temperature is
identical to the temperature of the fluid inside
the CSTR.
The rate of heat transfer from the exchanger to
the reactor is
For exothermic reactions, TgtTa2gtTa1 For
endothermic reactions, Ta1gtTa2gtT
14
An energy balance on the coolant fluid entering
and leaving the exchanger is
Assuming a quasi-steady for the coolant flow and
neglecting the accumulation term (i.e., dTa/dt0)
Cpc is the heat capacity of the coolant fluid TR
is the reference temperature.
for large coolant flow rates
15
(No Transcript)
16
(No Transcript)
17
Example 8-8
Propylene glycol is produced by the hydrolysis of
propylene oxide
Over 800 million pounds of propylene glycol were
produced in 2004 and the selling price was
approximately 0.68 per pound. Propylene glycol
makes up about 25 of the major derivatives of
propylene oxide. The reaction takes place readily
at room temperature when catalyzed by sulfuric
acid. You are the engineer in charge of an
adiabatic CSTR producing propylene glycol by this
method. Unfortunately, the reactor is beginning
to leak, and you must replace it. (You told your
boss several times that sulfuric acid was
corrosive and that mild steel was a poor material
for construction.) There is a nice-looking
overflow CSTR of 300-gal capacity standing idle
it is glass-lined, and you would like to use
it. You are feeding 2500 lb/h (43.04 lb mol/h) of
propylene oxide (P.O.) to the reactor. The feed
stream consists of (1) an equivolumetric mixture
of propylene oxide (46.62 ft3/h) and methanol
(46.62 ft3/h), and (2) water containing 0.1 wt
H2SO4. The volumetric flow rate of water is 233.1
ft3/h, which is 2.5 times the methanol-P.O. flow
rate. The corresponding molar feed rates of
methanol and water 71.87 and 802.8 lb mol/h,
respectively. The water-propylene-oxide-methanol
mixture undergoes a slight decrease in volume
upon mixing (approximately 3), but you neglect
this decrease in your calculations. The
temperature of both feed streams is 58?F prior to
mixing, but there is immediate 17?F temperature
rise upon mixing of the two feed streams caused
by the heat of mixing. The entering temperature
of all feed streams is thus taken to be 75?F
(Figure E8-8.1).
18
Furusawa et. al. state that under conditions
similar to those at which you are operating, the
reaction is first-order in propylene oxide
concentration and apparent zero-order in excess
of water with the specific reaction rate
The units of E are Btu/lb mol.
There is an important constraint on your
operation. Propylene oxide is a rather
low-boiling substance. With the mixture you are
using, you feel that you can not exceed an
operating temperature of 125?F, or you will lose
too much oxide by vaporization through the vent
system. Can you use the idle CSTR as a
replacement for the leaking one if it will be
operated adiabatically? If so, what will be the
conversion of oxide to glycol?
19
Solution
In this problem, neither the exit conversion nor
the temperature of the adiabatic reactor is
given. By application of the material and energy
balances we can solve two equation with two
unknowns (X and T). Solving these coupled
equations we determine the exit concentration and
temperature for the glass-lined reactor to see if
it can be used to replace the present reactor.
1. Mole Balance (design equation)
4. Combining
2. Rate Law
3. Stoichiometry (liquid phase)
20
5. Energy Balance
6. Calculations
a. Heat of reaction at temperature T
b. Stoichiometry
21
c. Evaluate mole balance terms
d. Evaluate energy balance terms
22
X0.85 T613?R
We observe from this plot that the only
intersection point is at 85 conversion and
613?R. At this point, both the energy balance and
mole balance are satisfied. Because the
temperature must remain below 125?F (585?R), we
cannot use the 300-gal reactor as it is now.
23
Example 8-9
A cooling coil has been located in equipment
storage for use in the hydration of propylene
oxide discussed in Example 8-8. The cooling coil
has 40 ft2 of cooling surface and the cooling
water flow rate inside the coil is sufficiently
large that a constant coolant temperature of 85?F
can be maintained. A typical overall
heat-transfer coefficient for such a coil is 100
Btu/h?ft2??F. Will the reactor satisfy the
previous constraint of 125?F maximum temperature
if the cooling coil is used?
Solution
24
X0.36 T564?R
25
Multiple Steady States
Consider the steady-state operation of a CSTR in
which a first-order reaction is taking place.
heat-generated term
heat-removed term
26
Heat-Removed Term, R(T)
We see that R(T) increases linearly with
temperature, with slope CP0(1?). As the entering
temperature T0 is increased, the line retains the
same slope but shifts to the right as shown in
Figure 8-14.
If one increase ? by either decreasing the molar
flow rate FA0 or increasing the heat-exchange
area, the slope increases and the ordinate
intercept moves to the left as shown in Figure
8-15, for conditions of TaltT0.
Note that if TagtTo, the intercept will move to
the right as ? increases.
27
Heat-Generated Term, G(T)
first-order liquid reaction
second-order liquid reaction
Low E
high E
low T
high T
28
Ignition-Extinction Curve
As the entering temperature is increased, the
steady-state temperature increases along the
bottom line until T05 is reached. Any fraction of
a degree increase in temperature beyond T05 and
the steady-state reactor temperature will jump up
to Ts11, as shown in Figure 8-20. The temperature
at which this jump occurs is called the ignition
temperature. If a reactor were operating at Ts12,
and we began to cool the entering temperature
down from T06, the steady-state reactor
temperature Ts3 would eventually be reached,
corresponding to an entering temperature T02. Any
slight decrease below T02 would drop the
steady-state reactor temperature to Ts2. As a
result, T02 is called the extinction temperature.
29
Ts8? Ts9
GgtR
T?
unstable steady state
Ts8
Ts8? Ts7
RgtG
T?
Ts9? Ts9
RgtG
T?
Ts9
Ts9? Ts9
GgtR
T?
locally stable
Ts7? Ts7
RgtG
T?
Ts7
Ts7? Ts7
GgtR
T?
30
in a CSTR operated adiabatically
The multiple steady-state temperatures were
examined by varying the flow rate over a range of
space times, ?, as shown in Figure 8-23. One
observes from this figure that at a space-time of
12 s, steady-state reaction temperatures of 4,
33, and 80?C are possible. If one were operating
on the higher steady-state temperature line and
the volumetric flow rates were steadily increase
(i.e., the space-time decreased), one notes that
if the space velocity dropped below about 7 s,
the reaction temperature would drop from 70?C to
2?C. The flow rate at which this drop occurs is
referred to as the blowout velocity.
31
Runaway Reactions in a CSTR
For a CSTR, we shall consider runway (ignition)
to occur when we move from the lower steady state
to the upper steady state. The ignition
temperature occurs at the point of tangency of
the heat removed curve to the heat-generated
curve. If we move slightly off this point of
tangency as shown in Figure 8-24, then runway is
said to have occurred.
For the heat-removed curve, the slope is
For the heat-generated curve, the slope is
32
If this difference between the reactor
temperature and Tc, ?Trc, is exceeded, transition
to the upper steady state will occur.
For many industrial reactions E/RT is typically
between 16 and 24, and the reaction temperatures
may be between 300 to 500 K. As a result, this
critical temperature difference ?Trc will be
somewhere around 15 to 30?C.
33
From Figure 8-25, we see that for a given value
of CP0(1?), if we were to increase the
entering temperature T0 from some low-value T01
(Tc1) to a higher entering temperature value T02
(Tc2), we would reach a point at which runway
would occur.
34
Nonisothermal Multiple Chemical Reactions
Energy Balance for Multiple Reactions in
Plug-Flow Reactors
q multiple reactions m species
Consider the following reaction sequence carried
out in a PFR
35
Example 8-10
The following gas-phase reaction occur in a PFR
Pure A is fed at a rate of 100 mol/s, a
temperature of 150?C, and a concentration of 0.1
mol/dm3. Determine the temperature and flow rate
profiles down the reactor.
36
Solution
Rate Law
Mole Balance
Relative Rates
Net Rates
37
Stoichiometry (gas phase ?P0)
Energy Balance
38
FA0
39
Energy Balance for Multiple Reactions in CSTR
q multiple reactions m species
For two parallel reactions,
40
Example 8-11
The elementary liquid-phase reactions
take place in a 10-dm3 CSTR. What are the
effluent concentrations for a volumetric feed
rate of 1000 dm3/min at a concentration of A of
0.3 mol/dm3? The inlet temperature is 283 K.
41
Solution
The reactions follow elementary rate laws
Mole Balance on Every Species
Rate Law
42
Energy Balance
43
(No Transcript)
44
Closure
Virtually all reactions that are carried out in
industry involve heat effects. This section
provides the basis to design reactors that
operate at steady state and involve heat
effects. To model these reactors, we simply add
another step to our algorithm this step is
the energy balance. Here it is important to
understand how the energy balance was applied to
each reaction type so that you will be able to
describe what would happen if you changed some
of the operating conditions (e.g.,
T0). Another major goal after studying this
section is to able to design reactors that
have multiple reactions taking place under
nonisothermal conditions.