A Third Example of Complex Equilibria - PowerPoint PPT Presentation
1 / 15
Title:
A Third Example of Complex Equilibria
Description:
I use X without the charge, to keep the problem general. ... See barium oxalate. for similar example. This simplifies to. Now use Ksp= [Br-] [Ag ... – PowerPoint PPT presentation
Number of Views:58
Avg rating:3.0/5.0
Title: A Third Example of Complex Equilibria
1
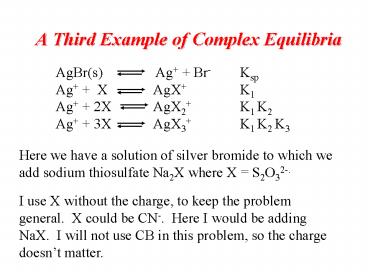
A Third Example of Complex Equilibria
AgBr(s) Ag Br- Ksp Ag X
AgX K1 Ag 2X
AgX2 K1 K2 Ag 3X AgX3
K1 K2 K3
Here we have a solution of silver bromide to
which we add sodium thiosulfate Na2X where X
S2O32-. I use X without the charge, to keep the
problem general. X could be CN-. Here I would
be adding NaX. I will not use CB in this
problem, so the charge doesnt matter.
2
Given
AgBr(s) Ag Br- Ksp Ag X
AgX K1 Ag 2X
AgX2 K1 K2 Ag 3X AgX3
K1 K2 K3
Since we are not using CB, how many MB equations
do we need to solve this problem.
Br- Ag AgX AgX2 AgX3
Cx AgX 2AgX2 3AgX3 X
Here Na 2Cx.
3
Given the MB equation
Br- Ag AgX AgX2 AgX3
use ais to simplify, so that right hand side is
only a functions of ais and Ag.
See barium oxalate for similar example.
This simplifies to
Now use Ksp Br- Ag
Ag2/a0
Rearranging gives
4
(1) (2) (3) (4)
Given Ksp Br- Ag
Ag2/a0
We can rearrange to find
Now solve for the Br- concentration using
With a little algebra one finds
What is left to do?
Note we are not done, since we know Cx not X.
5
Returning to the initial equations
AgBr(s) Ag Br- Ksp Ag X
AgX K1 Ag 2X
AgX2 K1 K2 Ag 3X AgX3
K1 K2 K3
Br- Ag AgX AgX2 AgX3
Cx AgX 2AgX2 3AgX3 X
The only equation we have not used is that for
Cx. This will be our master equation (one eqn
one unk).
Write the ME as a function of X.
6
The Master Equation
Cx AgX 2AgX2 3AgX3 X
We write the ME as a function of Ag and X
using the ai.
Now substitute
7
Complex Ion Formation Reviewed
PbI2(s) Pb2 2I- Pb2
I- PbI Pb2
2I- PbI2(aq) Pb2
3I- PbI3- Pb2 4I-
PbI42-
Ksp 7.9x10-9
1 2 3 4 5
b1K1 b2 K1 K2 b3 K1 K2 K3 b4 K1 K2 K3 K4
If one knows I- then one knows all the
concentrations, given that we are at equilibrium.
Lets make a plots just like we did for a weak
acid.
Pb2 Ksp I--2 PbI
b1Ksp I--1 PbI2(aq) b2 Ksp I-0
PbI3- b3Ksp I-1
8
Review of a-plots
Pbtot Pb2 PbI PbI2(aq) PbI3-
PbI42-
a0 Pb2 /Pbtot a1 PbI /Pbtot a2
PbI2(aq) /Pbtot a3 PbI3 - /Pbtot
Pb2 Ksp I--2 PbI
b1Ksp I--1 PbI2(aq) b2 Ksp I-0
PbI3 - b3Ksp I-1
9
a-plots continued
10
Do these formulas for PbI2 work for the AgXn
complexation problem as well?
Yes.
11
The master equation is
Plugging in the expressions for the ai we obtain
12
Numerical Results
13
In Excel one can vary X and calculate Cx and
all concentrations, and then plot results as a
function of Cx.
We would find that there were several regions.
One region would correspond to low values of X.
Ksp7.9x10-13, K16.6x108, K24.4x104, and K34.9
Finally we have
14
Homework Problems
- Derive expressions for the logAg as a function
of log(Cx) in regions 1 and 2. - Derive expressions for the logAgX as a
function of log(Cx) in regions 1 and 2.
15
Tiron Laboratory
You need to define a, b, and gs. You need to
write down mass balance equations for cL and
cFe. Write cFe equation as a function of Fe3
and a, b, and gs. Solve this equation for
Fe3. Write down cL as a function of Fe3 and
a, b, and gs. Write down cL as a function of and
a, b, and gs.