Stanley J. Szefler, MD - PowerPoint PPT Presentation
1 / 52
Title:
Stanley J. Szefler, MD
Description:
Helen Wohlberg and Herman Lambert Chair in ... BARGE. CLIC/PACT. PEAK. AIMS. MIA/MARS. CAMP. ACE. Question #1. 1. FEV1. 2. Bronchodilator reversibility ... – PowerPoint PPT presentation
Number of Views:96
Avg rating:3.0/5.0
Title: Stanley J. Szefler, MD
1
Stanley J. Szefler, MD
2
What Measures Can Be Useful To Monitor The
Natural History Of Pediatric Asthma?
- Stanley J. Szefler, MD
- Helen Wohlberg and Herman Lambert Chair in
Pharmacokinetics, - Head, Pediatric Clinic Pharmacology,
- National Jewish Medical and Research Center
- Professor of Pediatrics and Pharmacology,
University of Colorado Health Sciences Center
3
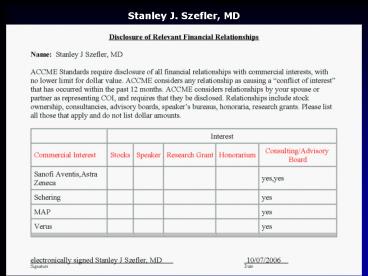
Disclosure
- Presenter Stanley J. Szefler, MD has documented
that he/she is a consultant for Astra Zeneca,
Genentech, Glaxo Smith Kline, MAP, Merck,
Novartis, Ross, Sanofi Aventis, Schering, Verus
and has resolved any identified conflicts of
interest - Presenter Stanley J. Szefler, MD has documented
that his/her presentation will not involve
discussion of unapproved or off-label,
experimental or investigational use.
4
Evolving Patterns in Asthma Management
5
Timeline
Original GuidelinesThrough Update on Selected
Topics 2002
6
Controller Therapy for Persistent Asthma in
Children and Adults
Severe Preferred High-dose ICS LABA and if
needed systemic corticosteroids
Moderate Preferred Low- to medium-doseICS
LABA or medium-dose ICS Alternative ? ICS to
med dose or low- to medium-dose ICS either
LTM or theophylline
Mild Preferred Low-dose ICS Alternative
cromolyn, LTM, nedocromil, or SR theophylline
ICS inhaled corticosteroid LTM leukotriene
modifier SR sustained-release LABA
long-acting ?2-agonist. For children aged ?5 y
and adults For children ?5 y.
NAEPP. Guideline Update 2002. J Allergy Clin
Immunol 2002110(pt 2)S141-219.
7
Clinical Questions
- Do children differ from adults in the underlying
pathophysiologic features that present as the
diagnosis of asthma? - Should there be a different approach to treatment
for children as compared to adults? - Do we need new medications specific for childhood
asthma?
8
Unique Features of Childhood Asthma
- Asthma can present in early childhood as acute
exacerbations. - Difficult to assess mild, persistent symptoms in
children. - Early asthma may primarily represent airway
inflammation while asthma of longer duration has
added features of airway remodeling. - Precautions of early intervention include drug
effect on growth and development. - Children may require age-specific methods to
administer inhaled medications.
9
Potential Approaches to Improving Asthma Control
- Early intervention
- Combination therapy
- Biomarkers
- Genetics
- Immunomodulators
10
NIH Network Activity
- NHLBI Childhood Asthma Management Program (CAMP)
- NHLBI Asthma Clinical Research Network (ACRN)
- NHLBI Childhood Asthma Research and Education
(CARE) Network - NIAID Inner City Asthma Consortium (ICAC)
11
STEPWISE APPROACH FOR MANAGING ASTHMA
S T E D P O W N
SLIC
Step 4 (Severe)
SOCS
AIMS
BAGS
MIA/MARS
CAMP
IMPACT
ACE
CLIC/PACT
PEAK
MICE/DICE
INTERMITTENT
PERSISTENT
BARGE
12
Question 1
What is the best pulmonary function measure to
follow the natural history of asthma in children?
1. FEV1 2. Bronchodilator reversibility 3.
FEV1percent predicted 4. FEV1/FVC
13
CAMP Baseline Asthma Features
- Mild to moderate persistent asthma
- Age 5-12 yrs
- Asthma duration 5.0 yrs
- Asthma burden during run-in
- symptoms 4.6 days/wk
- pre-BD FEV1 94 predicted
- pre-BD FEV1/FVC 80
- FEV1 PC20 1.1 mg/ml
CAMP trial began in 1993
14
CAMP Continuation Study (CAMPCS)
- 4 year post CAMP Trial follow-up to determine
- Evolution of airflow obstruction
- Long-term effects of anti-inflammatory treatments
on lung growth - Mean CAMP/CAMPCS follow-up 8.8 yrs (cohort
ranged from 12 to 22 yrs at end of CAMPCS)
CAMPCS began in 1999
15
Strengths of CAMPCS to Study Lung Growth in
Asthmatic Children
- Largest and most comprehensive longitudinal study
of mild to moderate persistent asthma in children - First long-term controlled clinical trial to
determine effect of anti-inflammatory treatment
on long-term lung growth
Strunk R and CAMP Research Group. JACI, 2006
16
Major Comparisons
- Lung growth in mild to moderate asthma compared
to normal - Comparison of CAMP to 6 Cities Study
- Effect of inhaled anti-inflammatory treatment on
lung growth - Intention to treat analysis
17
Lung Growth in Mild-Moderate Asthma Compared to
Normal
- Longitudinal data obtained from normal children
are needed for comparison to CAMP - Avoid age, period, and cohort effects inherent in
cross-sectional data - Pre-BD spirometry values used
- Gold standard for trials and observational
studies - Reflect airway tone
- Correlate with long-term outcomes of lung disease
- No post-BD values available from normal children
18
6 Cities Study
- 6 cities in different states (KS, MA, MO, OH, TN,
WI) with 13,781 enrolled - Annual examinations from 1974 - 1989
- Spirometry
- Weight and standing height
- Symptoms and asthma diagnosis
19
Normal Comparison Group
- 6 Cities sub-cohort
- Children without asthma or symptoms of asthma
- Sub-cohort characteristics
- Number in sub-cohort 5630
- Percent male 51.1
- Percent minorities 10.9
- Age
- Mean 8.1 yrs (range 4-15) at initial exam
- Mean 13.2 yrs (range 5-20) at final exam
20
Statistical MethodsCAMP and 6 Cities Comparison
- Separate analyses for boys and girls
- Multiple linear regression models
- Outcomes FEV1(L), FVC(L), FEV1/FVC ()
- Predictors
- Study indicator (CAMP vs. 6 Cities)
- Age indicators (splines) for ages 5 18 years
- Height height, height2
- Race indicator (white vs. minority)
- Interaction terms study X age indicators
21
FEV1 (L) and FVC (L) -- BoysCAMP vs 6 Cities
Sub-cohort
FEV1
4.0
(L)
3.0
1
Plt.0001
FEV
Plt.0001
2.0
1.0
6
8
10
12
14
16
18
Age (years)
- FEV1, FVC (pre-BD) are adjusted for height and
race. - 6 Cities sub-cohort are subjects never reporting
wheeze or asthma on any exam.
Strunk R and CAMP Research Group. JACI, 2006
22
FEV1 (L) and FVC (L) -- Girls CAMP vs 6 Cities
Sub-cohort
Plt.0001
Plt.0001
- FEV1, FVC (pre-BD) are adjusted for height and
race. - 6 Cities sub-cohort are subjects never reporting
wheeze or asthma on any exam.
Strunk R and CAMP Research Group. JACI, 2006
23
FEV1/FVC () CAMP vs 6 Cities Sub-cohort
Plt.0001
Plt.0001
- FEV1/FVC (pre-BD) adjusted for height and race.
- 6 Cities sub-cohort are subjects never reporting
wheeze or asthma on any exam.
Strunk R and CAMP Research Group. JACI, 2006
24
Effects of Mild-Moderate Persistent Asthma on
Lung Growth Comparison of CAMP to 6 Cities
Study1
- FEV1 growth reduced
- FVC growth increased
- Occur in both genders and become more apparent
with increasing age - May reflect differential growth of lung
parenchyma (FVC) compared to airways (FEV1)
25
Effects of Mild-Moderate Persistent Asthma on
Lung Growth Comparison of CAMP to 6 Cities
Study2
- Effect of asthma on FEV1/FVC most prominent lung
growth finding - Indicates airway obstruction that becomes more
apparent with increasing age - May indicate remodeling
- May be related to development of fixed airflow
obstruction in older adults
26
Effects of Mild-Moderate Persistent Asthma on
Lung Growth Comparison of CAMP to 6 Cities
Study3
- Differential growth of lung parenchyma and
airways in asthmatics - Growth of airways may be slower due to
inflammation - Growth of parenchyma may be greater to allow
stretch on inflamed airways to minimize closure
27
Implications Of These Findings
- Mild-moderate persistent asthma in children is
associated with abnormalities in lung growth and
increasing obstruction identified by the FEV1/FVC
ratio - Asthma in children may appear to be only
mild-moderate symptomatically, but significant
lung function abnormalities are present and
appear to be increasing with increasing age - Treatment with anti-inflammatory drugs for 4.3
years does not influence these effects
28
Improving Asthma Control Biomarkers and Genetics
- Can they be useful for -
- Diagnosing asthma?
- Predicting asthma severity?
- Predicting treatment response?
- Monitoring treatment response?
- Preventing adverse effects to treatment?
29
Improving Asthma Control
- Biomarkers
- Predictors of treatment response?
- Monitors of treatment response?
30
Improving Asthma Control
- Goals of long-term control therapy
- Prevent symptoms
- Improve pulmonary function
31
Question 2
What is the best predictor of pulmonary response
to inhaled corticosteroids in children and adults?
1. Exhaled nitric oxide 2. Bronchodilator
reversibility 3. FEV1 percent predicted 4.
FEV1/FVC
32
Study Timeline
Assessment/ Characterization
Treatment Phase
Mt
Mt
FP
FP
Randomization
Mt
Mt
FP
FP
Visit
1
2
3
4
5
6
Week
-1
0
4
8
12
16
Consent Asthma Hx eNO Spirometry BD
response Biomarkers Genetics Diary and PFM
Review diary eNO Spirometry Methacholine Skin
testing
Review diary eNO Spirometry
33
Primary Outcome FEV1 Response
Concordance Correlation 0.55 (0.43, 0.65)
50
Mt alone n6 (5)
Both n22 (17)
gt7.5 Mt Response
40
30
20
10
FEV1 Change with Mt
0
-10
-20
FP alone n29 (23)
Line of identity
-30
Neither n69 (55)
-40
gt7.5 FP Response
-50
-50
-40
-30
-20
-10
0
10
20
30
40
50
FEV1 Change with FP
Ref. Szefler SJ and CARE Network. JACI
2005115233-42.
34
FEV1 Response 7.5Median Baseline
Characteristics
Ref. Szefler SJ and the CARE Network. J Allergy
Clin Immunol 2005115233-42.
35
FEV1 Response 7.5Odds Ratio
Ref. Szefler SJ and the CARE Network. J Allergy
Clin Immunol 2005115233-42.
36
Individual Difference in FEV1 Response
Better Response to Fluticasone Propionate (n75)
Participants
Better Response to Montelukast (n24)
Difference in FEV1 Response, of Baseline
(Fluticasone Propionate Montelukast)
Ref. Szefler SJ and the CARE Network. JACI
2005115 233-42.
37
Differential Response Analysis
Greater response to fluticasone over montelukast
was associated with
- Higher bronchodilator use
- Greater response to bronchodilator
- Higher exhaled nitric oxide
- Higher serum eosinophilic cationic protein
- Lower pre-bronchodilator FEV1 percent predicted
- Lower FEV1/FVC
- Lower methacholine PC20
Ref. Szefler SJ and the CARE Network. J Allergy
Clin Immunol 2005115233-42.
38
Individual Difference in Asthma-Free Days
Response
Better Response to Fluticasone Propionate
(n36)
Participants
Better Response to Montelukast (n15)
Ref. Zeiger RS and the CARE Network. JACI
2006117 45-52.
Difference in Asthma Free Days Response (Fluticaso
ne Propionate Montelukast)
39
Differential Response AnalysisAsthma-Free Days
Greater response to fluticasone over montelukast
was associated with the following baseline asthma
feature
- Higher eNO
Ref. Zeiger RS and the CARE Network. JACI
2006117 45-52.
40
Improving Asthma Control
- Goals of long-term control therapy
- Prevent symptoms
- Improve pulmonary function
- Reduce inflammation
41
Improving Asthma Control
- Biomarkers
- Predictors of treatment response?
- Monitors of treatment response?
42
Question 3
What markers have been tested as tools to adjust
asthma therapy during ongoing managment?
1. Airway hyperresponsiveness to mannitol 2.
Sputum neutrophils 3. Sputum eosinophilic
cationic protein 4. Exhaled nitric oxide
43
Monitoring ICS Use
- Are there ways to optimize the use of ICS
- Targeting biologic markers in asthma
- Airway hyperresponsiveness
- Sputum eosinophils
- Exhaled nitric oxide (eNO)
44
Treatment Strategy and Measuresof Response
Guidelines Approachvs Inflammation-Based Approach
120
BTS management group
Sputum management group
100
80
Severe Exacerbations
60
40
20
0
0
1
2
3
5
4
6
7
12
11
10
9
8
Number of Exacerbations
Time (mo)
BTS group
0
12
19
26
35
59
75
93
109
Sputum group
0
1
4
7
12
17
21
30
35
Green RH et al. Lancet 2002 3601715-21.
45
Rates of Exacerbation
Smith, A. et al. N Engl J Med 20053522163-2173
46
Question 4
Children differ from adults in which of the
following asthma characteristics
1. Children tend to have higher FEV1 predicted
than adults 2. Children have a lesser degree of
asthma inflammation than adults 3. Children have
higher exhaled nitric oxide than adults 4.
Children have greater airway hyperresponsiveness
than adults
47
Similarities
- Variable response to inhaled corticosteroids
- Potential predictors of steroid response
- Impact of Arg/Arg genotype on drug response
48
Potential Differences
- Technique for measurement of pulmonary function
- Pulmonary function higher in children than adults
- Predictors of steroid response
- - eNO in children
- - Bronchodilator response in adults
- Predictors of LTRA response
- - uTRAs in children
- Level of plateau response may differ due to
structural features (hyperinflation in children
vs. airway remodeling in adults) - Effect of medications on growth and development
49
Questions for Childhood Asthma
- Appropriate intervention in young children?
- Treatments that alter the natural history of
asthma? - Management of asthma poorly controlled on
low-dose ICS? - Role of as needed combination therapy in
management of children? - Management of moderate persistent asthma?
50
Pediatric AsthmaIs It Different?
- ABSOLUTELY!!!
- Different from adults
- Different among children
- Different approach to treatment
- Different concerns regarding safety of
intervention
51
Natural History of Asthma
Proposed individualized approach
- Dramatically change your overall approach to
asthma management - Analyze patient characteristics including
genetics to identify risk features and develop
management plan - Use biomarkers to predict and monitor treatment
response - Follow course of asthma
- - Monitor pulmonary function over time
- FEV1 predicted, FEV1/FVC
- - Record and monitor treatment to achieve
asthma control as defined by patient and
physician
52
Asthma Management
Individualized Approach
- Utilize asthma characteristics, biomarkers, and
genetics to profile asthma prognosis and
severity. - Select medications based on driving factors of
disease presentation, predictors of response, and
risks of poor control. - Monitor response and assess reasons for treatment
failure. - Develop proactive approach and adjust therapy
according to definition of control and risk.