Fundamentals of Spectrophotometry - PowerPoint PPT Presentation
1 / 43
Title:
Fundamentals of Spectrophotometry
Description:
Spectrophotometer. 1.) Basic Design ... to make absorbance or transmittance measurements is known as a spectrophotometer ... 2.) Types of Spectrophotometers ... – PowerPoint PPT presentation
Number of Views:1282
Avg rating:3.0/5.0
Title: Fundamentals of Spectrophotometry
1
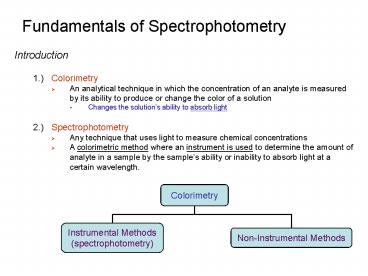
Fundamentals of Spectrophotometry
- Introduction
- 1.) Colorimetry
- An analytical technique in which the
concentration of an analyte is measured by its
ability to produce or change the color of a
solution - Changes the solutions ability to absorb light
- 2.) Spectrophotometry
- Any technique that uses light to measure chemical
concentrations - A colorimetric method where an instrument is used
to determine the amount of analyte in a sample by
the samples ability or inability to absorb light
at a certain wavelength.
Colorimetry
Instrumental Methods (spectrophotometry)
Non-Instrumental Methods
2
Fundamentals of Spectrophotometry
- Introduction
- 3.) Illustration
- Measurement of Ozone (O3) Above South Pole
- O3 provides protection from ultraviolet radiation
- Seasonal depletion due to chlorofluorocarbons
O3 cycle
Chain Reaction Depletion of O3
Spectra analysis of O3
3
Fundamentals of Spectrophotometry
- Properties of Light
- 1.) Particles and Waves
- Light waves consist of perpendicular, oscillating
electric and magnetic fields - Parameters used to describe light
- amplitude (A) height of waves electric vector
- Wavelength (l) distance (nm, cm, m) from peak to
peak - Frequency (n) number of complete oscillations
that the waves makes each second - Hertz (Hz) unit of frequency, second-1 (s-1)
- 1 megahertz (MHz) 106s-1 106Hz
4
Fundamentals of Spectrophotometry
- Properties of Light
- 1.) Particles and Waves
- Parameters used to describe light
- Energy (E) the energy of one particle of light
(photon) is proportional to its frequency
where E photon energy (Joules) n frequency
(sec-1) h Plancks constant (6.626x10-34J-s)
As frequency (n) increases, energy (E) of light
increases
5
Fundamentals of Spectrophotometry
- Properties of Light
- 1.) Particles and Waves
- Relationship between Frequency and Wavelength
- Relationship between Energy and Wavelength
where c speed of light (3.0x108 m/s in
vacuum)) n frequency (sec-1) l wavelength
(m)
where (1/l) wavenumber
As frequency (l) decreases, energy (E) of light
increases
6
Fundamentals of Spectrophotometry
- Properties of Light
- 2.) Types of Light The Electromagnetic Spectrum
- Note again, energy (E) of light increase as
frequency (n) increases or wavelength (l)
decreases
7
Fundamentals of Spectrophotometry
- Properties of Light
- 2.) Types of Light The Electromagnetic Spectrum
8
Fundamentals of Spectrophotometry
- Absorption of Light
- 1.) Colors of Visible Light
- Many Types of Chemicals Absorb Various Forms of
Light - The Color of Light Absorbed and Observed passing
through the Compound are Complimentary
9
Fundamentals of Spectrophotometry
- Absorption of Light
- 2.) Ground and Excited State
- When a chemical absorbs light, it goes from a low
energy state (ground state) to a higher energy
state (excited state) - Only photons with energies exactly equal to the
energy difference between the two electron states
will be absorbed - Since different chemicals have different electron
shells which are filled, they will each absorb
their own particular type of light - Different electron ground states and excited
states
Energy required of photon to give this
transition DE E1 - Eo
10
Fundamentals of Spectrophotometry
- Absorption of Light
- 3.) Beers Law
- The relative amount of a certain wavelength of
light absorbed (A) that passes through a sample
is dependent on - distance the light must pass through the sample
(cell path length - b) - amount of absorbing chemicals in the sample
(analyte concentration c) - ability of the sample to absorb light (molar
absorptivity - e)
Increasing Fe2
Absorbance is directly proportional to
concentration of Fe2
11
Fundamentals of Spectrophotometry
- Absorption of Light
- 3.) Beers Law
- The relative amount of light making it through
the sample (P/Po) is known as the transmittance
(T)
Percent transmittance
T has a range of 0 to 1, T has a range of 0 to
100
12
Fundamentals of Spectrophotometry
- Absorption of Light
- 3.) Beers Law
- Absorbance (A) is the relative amount of light
absorbed by the sample and is related to
transmittance (T) - Absorbance is sometimes called optical density
(OD)
A has a range of 0 to infinity
13
Fundamentals of Spectrophotometry
- Absorption of Light
- 3.) Beers Law
- Absorbance is useful since it is directly related
to the analyte concentration, cell pathlength and
molar absorptivity. - This relationship is known as Beers Law
where A absorbance (no units) e molar
absorptivity (L/mole-cm) b cell pathlength
(cm) c concentration of analyte (mol/L)
Beers Law allows compounds to be quantified by
their ability to absorb light, Relates directly
to concentration (c)
14
Fundamentals of Spectrophotometry
- Absorption of Light
- 4.) Absorption Spectrum
- Different chemicals have different energy levels
- different ground vs. excited electron states
- will have different abilities to absorb light at
any given wavelength - Absorption Spectrum plot of absorbance (or e)
vs. wavelength for a compound - The greater the absorbance of a compound at a
given wavelength (high e), the easier it will be
to detect at low concentrations
15
Fundamentals of Spectrophotometry
- Absorption of Light
- 4.) Absorption Spectrum
- By choosing different wavelengths of light (lA
vs. lB) different compounds can be measured
lA
lB
16
Fundamentals of Spectrophotometry
- Spectrophotometer
- 1.) Basic Design
- An instrument used to make absorbance or
transmittance measurements is known as a
spectrophotometer
17
Fundamentals of Spectrophotometry
- Spectrophotometer
- 1.) Basic Design
- Light Source provides the light to be passed
through the sample - Tungsten Lamp visible light (320-2500 nm)
- Deuterium Lamp ultraviolet Light (160-375 nm)
- based on black body radiation heat solid
filament to glowing, light emitted will be
characteristic of temperature more than nature of
solid filament
Low pressure (vacuum)
Tungsten Filament
In presence of arc, some of the electrical energy
is absorbed by D2 (or H2) which results in the
disassociation of the gas and release of
light D2 Eelect ? D2 ? D D hn
(light produced) Excited state
18
Fundamentals of Spectrophotometry
- Spectrophotometer
- 1.) Basic Design
- Wavelength Selector (monochromator) used to
select a given wavelength of light from the light
source - Prism
- Filter
19
Fundamentals of Spectrophotometry
- Spectrophotometer
- 1.) Basic Design
- Wavelength Selector (monochromator) used to
select a given wavelength of light from the light
source - Reflection or Diffraction Grating
20
Fundamentals of Spectrophotometry
- Spectrophotometer
- 1.) Basic Design
- Sample Cell sample container of fixed length
(b). - Usually round or square cuvet
- Made of material that does not absorb light in
the wavelength range of interest - Glass visible region
- Quartz ultraviolet
- NaCl, KBr Infrared region
21
Fundamentals of Spectrophotometry
- Spectrophotometer
- 1.) Basic Design
- Light Detector measures the amount of light
passing through the sample. - Usually works by converting light signal into
electrical signal
Process a) light hits photoemissive
cathode and e- is emitted. b) an emitted
e- is attracted to electrode 1
(dynode 1), which is 90V more positive.
Causes several more e- to be emitted.
c) these e- are attracted to dynode 2, which is
90V more positive then dynode
1, emitting more e-. d)
process continues until e- are collected at
anode after amplification
at 9 dynodes. e) overall voltage between
anode and cathode is
900V. f) one photon produces 106 107
electrons. g) current is amplified and
measured
Photomultiplier tube
22
Fundamentals of Spectrophotometry
- Spectrophotometer
- 2.) Types of Spectrophotometers
- Single-Beam Instrument sample and blank are
alternatively measured in same sample chamber.
23
Fundamentals of Spectrophotometry
- Spectrophotometer
- 2.) Types of Spectrophotometers
- Double-Beam Instrument
- Continuously compares sample and blank
- Automatically corrects for changes in electronic
signal or light intensity of source
24
Fundamentals of Spectrophotometry
- Chemical Analysis
- 1.) Calibration
- To measure the absorbance of a sample, it is
necessary to measure Po and P ratio - Po the amount of light passing through the
system with no sample present - P the intensity of light when the sample is
present - Po is measured with a blank cuvet
- Cuvet contains all components in the sample
solution except the analyte of interest - P is measured by placing the sample in the cuvet.
- To accurately measure an unknown concentration,
obtain a calibration curve using a range of known
concentrations for the analyte
25
Fundamentals of Spectrophotometry
- Chemical Analysis
- 2.) Limitations in Beers Law
- Results in non-linear calibration curve
- At high concentrations, solute molecules
influence one another because of their proximity - Molar absorptivity changes
- Affect on equilibrium, (HA and A- have difference
absorption) - Analyte properties change in different solvents
- Errors in reproducible positioning of cuvet
- Also problems with dirt fingerprints
- Instrument electrical noise
Keep A in range of 0.1 1.5 absorbance units (80
-3T)
26
Fundamentals of Spectrophotometry
- Chemical Analysis
- 3.) Precautions in Quantitative Absorbance
Measurements - Choice of Wavelength
- Choose a wavelength at an absorption maximum
- Minimizes deviations from Beers law, which
assumes e is constant - Pick peak in absorption spectrum where analyte is
only compound absorbing light - Or choose a wavelength where the analyte has the
largest difference in its absorbance relative to
other sample components
Bad choice for either compound (a) or (b)
Best choice compound (b)
Best choice compound (a)
27
Fundamentals of Spectrophotometry
- Chemical Analysis
- 4.) Example
A 3.96x10-4 M solution of compound A exhibited an
absorbance of 0.624 at 238 nm in a 1.000 cm
cuvet. A blank had an absorbance of 0.029. The
absorbance of an unknown solution of compound A
was 0.375. Find the concentration of A in the
unknown.
28
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 1.) Molecule Promoted to a More Energetic Excited
State - Absorption of UV-vis light results in an electron
promoted to a higher energy molecular orbital
- s ? s
- transition in vacuum UV
- n ? s
- saturated compounds with non-bonding
electrons - n ? p, p ? p
- requires unsaturated functional groups
- (eq. double bonds)
- most commonly used, energy good range
for UV/Vis
29
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 1.) Molecule Promoted to a More Energetic Excited
State - Geometrical Structure of the Excited State will
Differ from the Ground State
Ground State
Excitation of an electron to the pi antibonding
orbital (p) in formaldehyde produces repulsion
instead of attraction between the carbon and
oxygen atom
Excited State
30
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 1.) Molecule Promoted to a More Energetic Excited
State - Two Possible Transitions in Excited State
- Single state electron spins opposed
- Triplet state electron spins are parallel
- In general, triplet state has lower energy than
singlet state - Singlet to Triplet transition has a very low
probability - Singlet to Singlet Transition are more probable
31
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 2.) Infrared and Microwave Radiation
- Not energetic enough to induce electronic
transition - Change vibrational, translational and rotational
motion of the molecule - The entire molecule and each atom can move along
the x, y, z-axis - When correct wavelength is absorbed,
- Oscillations of the atom vibration is increased
in amplitude - Molecule rotates or moves (translation) faster
Vibrational States of Formaldehyde
Energy Electronic gtgt Vibrational gt Rotational
32
symmetric
asymmetric
In-plane scissoring
Out-of-plane wagging
In-plane rocking
Out-of-plane twisting
33
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 3.) Combined Electronic, Vibrational and
Rotational Transitions - Absorption of photon with sufficient energy to
excite an electron will also cause vibrational
and rotational transitions - There are multiple vibrational and rotational
energy levels associated with each electronic
state - Excited vibrational and rotational states are
lower energy than electronic state - Therefore, transition between electronic states
can occur between different vibrational and
rotational states
Vibrational and rotational states associated with
an electronic state
34
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 4.) Relaxation Processes from Excited State
- There are multiple possible relaxation pathways
- Vibrational, Rotational relaxation occurs through
collision with solvent or other molecules - energy is converted to heat (radiationless
transition) - Electronic relaxation occurs through the release
of a photon (light)
35
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 4.) Relaxation Processes from Excited State
- Internal conversion transition between singlet
electronic states through overlapping vibrational
states - Intersystem crossing transition between a
singlet electronic state to a triplet electronic
state by overlapping vibrational states
36
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 4.) Relaxation Processes from Excited State
- Fluorescence emitting a photon by relaxing from
an excited singlet electronic states to a ground
singlet state - S1 ? So
- Phosphorescence emitting a photon by relaxing
from an excited triplet electronic states to a
ground singlet state - T1 ? So
37
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 5.) Fluorescence and Phosphorescence
- Relative rates of relaxation depends on the
molecule, the solvent, temperature, pressure,
etc. - Energy of Phosphorescence is less than the energy
of fluorescence - Phosphorescence occurs at a longer wavelengths
than fluorescence - Lifetime of Fluorescence (10-8 to 10-4 s) is very
short compared to phosphorescence (10-4 to 102 s) - Fluorescence and phosphorescence are relatively
rare
38
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 5.) Fluorescence and Phosphorescence
- Fluorescence and phosphorescence come at lower
energy than absorbance - Emission spectrum is roughly mirror image of
absorption spectrum
Color Change Due to Fluorescence at Higher
Wavelength
39
Fundamentals of Spectrophotometry
What Happens When a Molecule Absorbs Light?
- 5.) Fluorescence and Phosphorescence
- Emission spectrum are of lower energy or higher
wavelength because of the efficiency of
vibrational relaxation - Absorption to an excited vibrational state will
relax quickly to a ground vibrational state
before the electronic relaxation
40
Fundamentals of Spectrophotometry
- What Happens When a Molecule Absorbs Light?
- 5.) Fluorescence and Phosphorescence
- Also, differences in stability of excited and
ground state structure contribute to energy
difference
41
Fundamentals of Spectrophotometry
- Chemical Analysis
- 1.) Excitation and Emission Spectra
Excitation Spectra measure fluorescence or
phosphorescence at a fixed wavelength
while varying the excitation wavelength.
Emission Spectra measure fluorescence or
phosphorescence over a range of wavelengths
using a fixed varying excitation wavelength.
42
Fundamentals of Spectrophotometry
- Chemical Analysis
- 2.) Fluorescence and Phosphorescence Intensity
- At low concentration, emission intensity is
proportional to analyte concentration - Related to Beers law
- At high concentrations, deviation from linearity
occurs - Emission decreases because absorption increases
more rapidly - Emission is quenched ? absorption of excitation
or emission energy by analyte molecules in
solution
where k constant Po light intensity c
concentration of analyte (mol/L)
43
Fundamentals of Spectrophotometry
- Chemical Analysis
- 3.) Example
In formaldehyde, the transition n? p(T1) occurs
at 397 nm, and the n?p(S1) transition comes at
355 nm. What is the difference in energy (kJ/mol)
between the S1 and T1 states?