Simple to Complex Lifes Levels of Organization - PowerPoint PPT Presentation
1 / 25
Title:
Simple to Complex Lifes Levels of Organization
Description:
What's the difference between an atom and a molecule? ... To Understand the Big, You'd Better Know the Small. Atoms ... Some Atoms are Sociable, Others Aren't ... – PowerPoint PPT presentation
Number of Views:2169
Avg rating:3.0/5.0
Title: Simple to Complex Lifes Levels of Organization
1
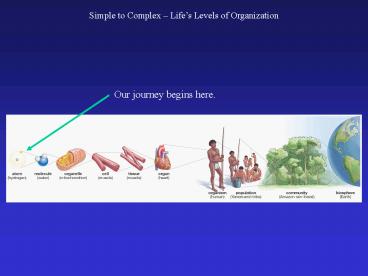
Simple to Complex Lifes Levels of Organization
2
Chemistry and Life
Whats the difference between an atom and a
molecule?
What makes atoms hold together to form molecules?
Does shape matter?
3
To Understand the Big, Youd Better Know the
Small
4
Atoms and Atomic Structure
Atoms are the smallest units of a chemical
element.
5
Atoms and Atomic Structure
In a simplified yet useful view of an atom,
electrons orbit a nucleus composed of protons and
neutrons.
6
Atoms Come in Different Forms Called Isotopes
Isotopes of a given element have the same number
of protons but different numbers of neutrons.
Many isotopes are unstable, making them
radioactive.
Radioactive isotopes (radioisotopes) play an
important role in health, medicine and biological
research.
7
Some Atoms are Sociable, Others Arent
Atoms want (are most stable) to have a filled
outer electron shell.
Atoms without a filled outer shell will share
electrons with other atoms to accomplish this
goal.
Filling outer electron shells controls which atom
will pair with which others and in what
combinations.
8
Filling Electron Shells
An important rule the innermost shell holds two
electrons subsequent shells hold 8 electrons.
9
Pairing for the Greater Good
When atoms come together by sharing electrons the
bond is a covalent bond.
H2
A molecule is formed when two or more atoms are
bound together covalently.
10
Drawing It Out
The sharing of a pair of electrons between atoms
(a covalent bond) is shown as
H-H (for H2)
or
H-O-H (for H2O)
or
11
Water - A Most Important Molecule
Note how bonding fills all outer electron shells.
12
Polar and Non-Polar Covalent Bonding
Some atoms have an equal affinity for electrons.
If so, the shared electrons spend equal amounts
of time around each atom and the covalent bond is
non-polar.
The covalent bonds of H2 and CH4 are non-polar
and so are the molecules.
13
Polar and Non-Polar Covalent Bonding
Some atoms have an unequal affinity for
electrons.
If so, the shared electrons spend more time
around one atom relative to another and the
covalent bond is polar.
Therefore, the covalent bonds of H2O are highly
polar and so is the molecule.
14
Polar and Non-Polar Covalent Bonding
15
Polar and Non-Polar Covalent Bonding
The polar versus non-polar distinction determines
which molecules will dissolve in a particular
solute.
For example, sugar dissolves in water, but fat
doesnt.
The general rule is like dissolves like.
16
Some Atoms Do Almost Anything to Fill Electron
Shells
Sodium donates a lonely electron to chlorine to
complete its outer electron shell. Chlorine is
only too happy to accept.
The result is ion formation.
An ion is an atom or molecule with one or more
full positive or negative charges.
17
Ions and Ion Formation
18
Ionic Bonds
Two oppositely charged ions bind together.
This type of chemical bond is an ionic bond.
Salts are solids held together by ionic bonds.
Ionic bonds are common and important in biology.
19
Hydrogen Bonding
Oxygen and nitrogen are much more hungry for
electrons than hydrogen.
Bonds between nitrogen or oxygen and hydrogen are
highly polar.
This allows bonds to form between partially
positive and partially negative atoms in
different or (in large molecules) the same
molecule.
The result is a hydrogen bond.
20
Hydrogen Bonding Gives Water Unique Properties
21
Relative Bond Strengths
gt
gt
22
Molecular Shape
Molecules have distinct shapes and shape
matters.
23
Molecular Shape
A regulatory protein molecule (yellow) binding to
DNA. Without complementary shapes, binding would
not occur.
24
Molecular Shape Matters
We perceive and distinguish odors because of the
particular shape of the odorant (the molecule we
smell) and receptor molecules on nose cells.
25
Biological Chemistry Takes Place in Solutions
Molecules are often described as hydrophilic
(water-loving) or hydrophobic (water-fearing) on
the basis of their solubility in water.

















![Self-Organization in Autonomous Sensor/Actuator Networks [SelfOrg] PowerPoint PPT Presentation](https://s3.amazonaws.com/images.powershow.com/3992822.th0.jpg?_=20130418128)












