The role of activating and inhibitory FcR - PowerPoint PPT Presentation
1 / 32
Title:
The role of activating and inhibitory FcR
Description:
Interaction between mAb Fc with Fc R is. required for anti-tumor activity. mutation ... Polio vaccine (Sabin): poliovirus grown in monkey cells ... – PowerPoint PPT presentation
Number of Views:103
Avg rating:3.0/5.0
Title: The role of activating and inhibitory FcR
1
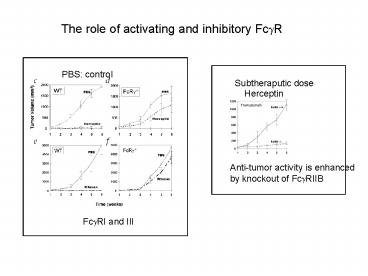
The role of activating and inhibitory Fc?R
PBS control
Subtheraputic dose
Herceptin
Anti-tumor activity is enhanced by knockout of
Fc?RIIB
Fc?RI and III
2
Interaction between mAb Fc with Fc?R is required
for anti-tumor activity
mutation
Asp265 to Ala265 (D265A)
4D5
Does not bind Fc?R
ADCC assay
NK cells
Breast carcinoma cells (51Cr)
Effector (NK) / target ratio
3
Direct arming mAb
mAb-toxin conjugate
Toxicity in clinical trials
Mylotarg
Humanized anti-CD33-calicheamicin
Treat CD33 acute myeloid leukemia
30 response rate
irradiation
Radioimmunoconjugates
131I (b ray)
Solid tumor
4
Problem with superagonist antibody drug
anti-CD28 mAb
Treat autoimmune disease?
CD4CD25Treg
CD28
CD4TH
Anti-CD28 Ab
Activation without TCR interaction
No activation in animal tests
Cytokine storm
Organ failure
CD4TH
Anti-CD28 Ab
Activation of CD4TH in humans
5
Immunization
Passive immunization preformed antibodies
transferred to recipient
Maternal IgG and IgA
Intravenous immune globulin (IVIG)
Immunoglobulin (mostly IgG) from pooled plasma of
blood donors
Animal anti-serum against toxins (Type III
hypersensitivity, serum sickness)
Transient protection
6
Active Immunization induction of immunity and
memory
Natural infection
Vaccine
Pathogen
Neutralizing antibody against
toxin
Especially important for Pathogen producing
toxin Pathogen with short incubation period
(influenza) Infection of irreplaceable tissues
(neurons by poliovirus)
CTL against infected cells (intracellular
infection)
Memory
7
Live-attenuated pathogens as vaccine
Grow the pathogen in non-human cells.
Polio vaccine (Sabin) poliovirus grown in monkey
cells
Measles vaccine rubella virus grown in duck
embryo cells
TB vaccine (BCG) Mycobacterium bovis grown in
media with bile.
Attenuated pathogen induce immunity without
causing disease.
May regain virulence through mutation.
May cause disease in immunodeficient patients.
8
Recombinant-vector vaccines
Attenuated Bacteria or virus
Gene encoding pathogen antigen
Attenuated Salmonella as carrier for genes
encoding tetanous toxin, Listeria
monocytogenes Bacillus anthracis, Leishmania
major, etc.
Recombinant adenovirus vectors, poxviruses,
alphaviruses
9
Inactivated pathogens and components as vaccines
Inactivated pathogen (by chemicals)
Salk polio vaccine polio virus inactivated by
formaldehyde
Bacterial polysaccharide capsules
Chemically inactivated toxins (toxoids)
Recombinant pathogen antigens
10
Influenza vaccine
HA binds to sialic acid- containing
carbohydrates on host cell
Acidification of endosome induces
conformational change in HA trimer
Type A, B, C based on NP and M1
Type A most common
Fusion of viral envelope and the endosomal
membrane
Subtype based on HA and NA HA H1-H16 NA N1-N9
e.g. H1N1, H5N1
11
Variability of influenza virus
HA
Minor mutations within the subtype
Avian influenza
Reassortment of genomic RNA
HA recognizes N-acetyl sialic acid-galactose
Human cells ?2,6 linkage
Duck cells ?2,3 linkage
Pig cells both ?2,6 and ?2,3 linkage
May serve as mixing vessel for Human and avian
viruses
Generation of new subtype (different HA, NA)
12
Current circulating influenza viruses
Direct transmission of avian virus to human
Lower respiratory tract contains a2,3 sialic
acid-galactose
1997 H5N1 infection in Hong Kong
2003-2004 H5N1 infection in Southeast Asia
No human to human transmission
2003 H9N2 infection in Hong Kong
2003 H7N7 infection in Netherlands, human
transmission
13
HA and pathogenicity
14
Current vaccine
Trivalent inactivated influenza vaccines (TIVs)
A strain (H1N1, H3N2), B strain
Chicken eggs
Chemical inactivation
New vaccine by reverse genetics
Viral replication proteins
PR8 grows well in eggs.
HA and NA of the pandemic strain
Required for efficient propagation in eggs
Basic residues deleted to reduce pathogenicity.
Simian kidney cells
Outer coat of the pandemic strain
Lack of pathogenicity (HA deletion)
Good growth in eggs
15
DNA vaccine
Injection of a plasmid into muscle leads to gene
expression.
plasmid
?-galactosidase gene
Injection into muscle
Muscle section Staining for ?-galactosidase
plasmid
human
Expression of pathogen antigen
Pathogen antigen gene
Immune response
16
Protection against influenza in mice
Science 259, 1745-1749 (1993)
CTL against infected cells
plasmid
a CTL from mice Injected with NP plasmid
Influenza NP cDNA
a
Lysis ()
b
b CTL from mice recovered from viral infection
c,d
c,d naïve mice or mice injected with control
plasmid
Effectortarget ratio
Antibody (IgG) against NP in serum
CTL
ConA IL2
Infected with influenza
CTL
51Cr
Lysis and release of 51Cr
Class II MHC-TH cell activation
17
Survival of DNA-immunized mice against viral
challenge
Mice with NP plasmid
Mice with control plasmid
No injection
18
Induction of CTL in humans by Malaria DNA vaccine
Science 282, 476-480 (1998)
plasmid
Plasmodium falciparum
P. falciparum antigen (PfCSP)
20 healthy malaria-naïve adults
CTL
Peripheral blood mononuclear cells (PBMC)
CTL assay against target cells
19
CTL activity
PfCSP Ag
CTL
Matched MHC
51Cr
Control Ag
Lysis 51Cr release
Non-matched MHC
control
peptide
PfCSP
Matched
MHC
Non-matched
20
Dendritic cells in the skin are the principle
APCs.
plasmid
Dendritic cells
Muscle cells keratinocytes
Cross-priming
MHC-I/antigen
MHC-II/antigen
Secretion Apoptotic cells
CD4T
B
CD8T
Lymph node
21
Response
Response -
DNA vaccine
Skin transplant
Response
Response -
time
0
12 hours
Langerhans cells in the skin
Langerhans cells have already migrated from the
skin
Removing muscle immediately after immunization
does not affect Immune response
22
DNA vaccine induces TH1 response.
plasmid
CTL
TLR9
TH1
IL12
CpG
APC
B cell
DNA vaccine is preferred for inducing immunity
against intracellular infection.
IgG2a
pXL2 (RSV DNA vaccine) TH1 response pXL0
(control)
Live RSV vaccine balanced TH1/TH2
Formalin inactivated RSV TH2 response
23
(No Transcript)
24
DNA vaccine expressing multiple antigens
DNA vaccine priming / boosting with other vaccines
25
Mucosal vaccine
Mucosal surface is the major site of pathogen
entry.
Mucosal vaccine induces both mucosal and systemic
immunity. Injected vaccines are poor inducers of
mucosal immunity.
M
DC
B
T
T
GC
Plasma cells, memory B cells
TH cells, CTL
26
Mucosal lymphocytes home to mucosal tissues.
Common mucosal immune system
Inductive site
Peyers patch
Effector site
Effector site
Other mucosal tissues
Other mucosal tissues
Plasma cells, CTL, TH cells Memory cells
Lymph node
Blood circulation
Blood circulation
27
Lymphocyte homing to mucosal tissue
Mucosal tissue-specific adhesion molecules and
chemokine/chemokine receptors
Mucosal effector site
a4b7 integrin
MADCAM1 (addressin)
Endothelial cells of venules in intestine
plasma cells
Epithelial cells of intestine and other mucosal
tissues
CCR10
CCL28
Mucosal induction site
a4b7 integrin
MADCAM1 (addressin)
Endothelial cells of venules in intestine
Effector T cells
Epithelial cells of intestine and other mucosal
tissues
CCR9
CCL25
28
Mucosal immunization also induces systemic
immunity.
M
Mucosal immunity
DC
B
T
T
GC
Mucosal lymphoid tissue
Lymph node
Systemic immunity
29
Mucosal plasma cells produce IgA.
Neutralization
Mucus
Dimeric IgA
Epithelial cells
IgA gt IgG
IgA
CTL
IgG
TH
Mucosal plasma cells
Blood circulation
Mucosal effector T and B cells
Blood circulation
Systemic immunity (lymph nodes, spleen)
IgG gt IgA
30
Preferential localization of mucosal immunity to
induction site
31
Challenges in mucosal vaccine
Degradation, dilution on mucosal surface
Live attenuated pathogen (polio vaccine, S. typhi
vaccine)
Use as recombinant vectors vaccines
Prevent tolerance
Mucosal adjuvant to induce danger signal
(activate DCs)
Mutated enterotoxins of bacteria (e.g. cholera
toxin)
PAMPs (e.g. CpG, flagellin)
32
Relevant part in book
Vaccine page 413-427