Einstein (1905): Energy of e.m.r. is quantised - PowerPoint PPT Presentation
1 / 139
Title:
Einstein (1905): Energy of e.m.r. is quantised
Description:
Mass ' of e.m.r. photon = m = h/lc. Overall conclusion: Light (e.m.r.) has both wave ... Compare the energies of photons emitted by two ... – PowerPoint PPT presentation
Number of Views:71
Avg rating:3.0/5.0
Title: Einstein (1905): Energy of e.m.r. is quantised
1
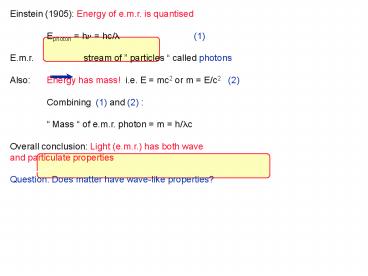
Einstein (1905) Energy of e.m.r. is quantised
Ephoton hn hc/l (1) E.m.r. stream of
particles called photons Also Energy has
mass! i.e. E mc2 or m E/c2 (2) Combining
(1) and (2) Mass of e.m.r. photon m
h/lc Overall conclusion Light (e.m.r.) has both
wave and particulate properties Question Does
matter have wave-like properties?
2
Does matter have wave-like properties?
3
Does matter have wave-like properties? Wave
Properties of Matter De Broglie (1925) Yes,
particularly for small masses
4
Does matter have wave-like properties? Wave
Properties of Matter De Broglie (1925) Yes,
particularly for small masses Similar equation
applies as for light m h/l.v or l
h/mv (v velocity of particle)
5
Does matter have wave-like properties? Wave
Properties of Matter De Broglie (1925) Yes,
particularly for small masses Similar equation
applies as for light m h/l.v or l
h/mv (v velocity of particle) For
an electron (m 9.1 x 10-28g, v 1.2 x
105m/s) l 6.1 x 10-9m
6
Does matter have wave-like properties? Wave
Properties of Matter De Broglie (1925) Yes,
particularly for small masses Similar equation
applies as for light m h/l.v or l
h/mv (v velocity of particle) For
an electron (m 9.1 x 10-28g, v 1.2 x
105m/s) l 6.1 x 10-9m For a cyclist (m 1 x
105g, v 3m/s) l 3.5 x 10-40m
7
- Energy of photon
- E hn hc/l
8
- Energy of photon
- E hn hc/l
- l 500 nm
9
- Energy of photon
- E hn hc/l
- l 500 nm
- E 6.626 x 10-34 x 3.00 x 109/ 500 x 10-9
- 3.97 x 10-18 J s m s-1 m-1
- 3.97 x 10-18 J
10
- Energy of photon
- E hn hc/l
- l 500 nm
- E 6.626 x 10-34 x 3.00 x 109/ 500 x 10-9
- 3.97 x 10-18 J s m s-1 m-1
- 3.97 x 10-18 J
- 100 W bulb emits 100 J s-1
- ? 100/ 3.97 x 10-18 2.5 x 1019 photons s-1.
11
- Summary so far
- On the atomic scale energy is transferred in
- discrete quantities (quanta)
- Light (e.m.r.) exhibits both wave and
particulate - behaviour
12
- Summary so far
- On the atomic scale energy is transferred in
- discrete quantities (quanta)
- Light (e.m.r.) exhibits both wave and
particulate - behaviour
- Matter, if small enough in mass, exhibits wave
- behaviour which is measurable
13
Line spectrum of sodium
400 550 700 nm
14
Line spectrum of sodium
400 550 700 nm
Line spectrum of hydrogen
400 550 700 nm
15
Line spectrum of sodium
400 550 700 nm
Line spectrum of hydrogen
400 550 700 nm
Balmer-Rydberg equation 1/l R1/m2 -
1/n2 m 1, n gt 1
16
- Bohr Theory of the Atom (1913)
- Developed a quantum model for the H atom
17
- Bohr Theory of the Atom (1913)
- Developed a quantum model for the H atom
- Electron moves around nucleus only in certain
- allowed circular orbits
18
- Bohr Theory of the Atom (1913)
- Developed a quantum model for the H atom
- Electron moves around nucleus only in certain
- allowed circular orbits
Allowed electron energy levels n 1 n 2 n
3, etc.
Nucleus
Electron in ground state atom
19
(No Transcript)
20
Energy put in
Energy given out
21
Energy given out
22
Energy given out
As a photon of given ?
23
Energy given out
As a photon of given ?
Other allowed e- transitions
Hydrogen emission spectrum
24
Energy put in
As a photon of given ?
Other allowed e- transitions
Hydrogen absorption spectrum
25
- Bohr theory
- Was based on a particulate view of the
electron - Worked reasonably well for the hydrogen atom
- But failed for all other elements
26
- Bohr theory
- Was based on a particulate view of the
electron - Worked reasonably well for the hydrogen atom
- But failed for all other elements
- Wave (Quantum) Mechanical Model of the Atom
- Schroedinger (1925)
- Applied de Broglies idea of a wave-like
electron - to electrons in atoms
27
- Bohr theory
- Was based on a particulate view of the
electron - Worked reasonably well for the hydrogen atom
- But failed for all other elements
- Wave (Quantum) Mechanical Model of the Atom
- Schroedinger (1925)
- Applied de Broglies idea of a wave-like
electron - to electrons in atoms
- Visualised electrons as standing waves,
existing - around the nucleus
28
Electron as a standing wave n 4
wavelengths Nucleus
Standing wave must have a whole number of
wavelengths to prevent destructive interference
29
- Quantization of e- energy levels by standing
- wave assumption
30
- Quantization of e- energy levels by standing
- wave assumption
- Derived a mathematical description for e-s as
- 3-d standing waves in atoms
31
- Quantization of e- energy levels by standing
- wave assumption
- Derived a mathematical description for e-s as
- 3-d standing waves in atoms
- H? E?
32
- Quantization of e- energy levels by standing
- wave assumption
- Derived a mathematical description for e-s as
- 3-d standing waves in atoms
- H? E?
- ?, the wave function, is a function spatial
- position of e-
- H, is a mathematical function which allows the
- calculation of the e- energy (Hamiltonian
- operator) and
- E, is the total energy of the electron
33
Fe atoms (blue) on a Cu crystal. Quantum energy
states of Cu electrons trapped inside the
circular Fe corral are imaged by STM as
waveforms (in red).
34
1
2
4
3
Making a quantum corral image sequence shows how
the scientists built the circular corral of iron
atoms in order to trap surface electron energy
states. (Courtesy of IBM)
35
Compare the energies of photons emitted by
two radio stations, operating at 92 MHz (FM) and
1500 kHz (MW)?
36
Compare the energies of photons emitted by
two radio stations, operating at 92 MHz (FM) and
1500 kHz (MW)? E hn 92 MHz 92 x 106 Hz gt
E 6.626 x 10-34 x 92 x 106 6.1 x 10-26J
37
Compare the energies of photons emitted by
two radio stations, operating at 92 MHz (FM) and
1500 kHz (MW)? E hn 92 MHz 2 x 106 Hz gt
E 6.626 x 10-34 x 2 x 106 1.33 x
10-27J 1500 kHz E 6.626 x 10-34 x 1.5 x 103
9.94 x 10-31J
38
The energy from radiation can be used to break
chemical bonds. Energy of at least 495 kJ mol-1
is required to break the oxygen-oxygen bond. What
is the wavelength of this radiation?
39
The energy from radiation can be used to break
chemical bonds. Energy of at least 495 kJ mol-1
is required to break the oxygen-oxygen bond. What
is the wavelength of this radiation? E
hc/l 495 x 103 J mol-1 ? 495 x 103 J mol-1/NA
8.22 x 10-19 J per molecule
40
- The energy from radiation can be used to break
chemical bonds. Energy of at least 495 kJ mol-1
is required to break the oxygen-oxygen bond. What
is the wavelength of this radiation? - E hc/l
- 495 x 103 J mol-1 ? 495 x 103 J mol-1/NA
- 8.22 x 10-19 J per molecule
- 6.626 x 10-34 x 3 x 108/ 8.22 x 10-19
- 242 x 10-9 m 242 nm.
41
Autumn 1999 2. The best available balances can
weigh amounts as small as 10-5 g. If you were to
count out water molecules at the rate of one per
second, how long would it take to count a pile
of molecules large enough to weigh 10-5 g?
42
Autumn 1999 2. The best available balances can
weigh amounts as small as 10-5 g. If you were to
count out water molecules at the rate of one per
second, how long would it take to count a pile
of molecules large enough to weigh 10-5 g? 1
molecule H2O has mass of 16 2 18 amu 1 mole
H2O has mass of 18 g ? 6.022 x 1023 molecules
43
Autumn 1999 2. The best available balances can
weigh amounts as small as 10-5 g. If you were to
count out water molecules at the rate of one per
second, how long would it take to count a pile
of molecules large enough to weigh 10-5 g? 1
molecule H2O has mass of 16 2 18 amu 1 mole
H2O has mass of 18 g ? 6.022 x 1023
molecules 10-5 g ? 10-5/18 moles 5.6 x 10-7
moles
44
Autumn 1999 2. The best available balances can
weigh amounts as small as 10-5 g. If you were to
count out water molecules at the rate of one per
second, how long would it take to count a pile
of molecules large enough to weigh 10-5 g? 1
molecule H2O has mass of 16 2 18 amu 1 mole
H2O has mass of 18 g ? 6.022 x 1023
molecules 10-5 g ? 10-5/18 moles 5.6 x 10-7
moles ? 5.6 x 10-7 x 6.022 x 1023 molecules
3.35 x 1017 molecules
45
Autumn 1999 2. The best available balances can
weigh amounts as small as 10-5 g. If you were to
count out water molecules at the rate of one per
second, how long would it take to count a pile
of molecules large enough to weigh 10-5 g? 1
molecule H2O has mass of 16 2 18 amu 1 mole
H2O has mass of 18 g ? 6.022 x 1023
molecules 10-5 g ? 10-5/18 moles 5.6 x 10-7
moles ? 5.6 x 10-7 x 6.022 x 1023 molecules
3.35 x 1017 molecules 3.35 x 1017 s
46
Autumn 2000 13. Hemoglobin absorbs light of
wavelength 407 nm. Calculate the energy (in J)
of one millimole of photons of this light.
47
Autumn 2000 13. Hemoglobin absorbs light of
wavelength 407 nm. Calculate the energy (in J)
of one millimole of photons of this light. E
hn hc/l 6.626 x 10-34 x 3 x 108 /407 x 10-9
J s m s-1 m-1
48
Autumn 2000 13. Hemoglobin absorbs light of
wavelength 407 nm. Calculate the energy (in J)
of one millimole of photons of this light. E
hn hc/l 6.626 x 10-34 x 3 x 108 /407 x 10-9
J s m s-1 m-1 4.88 x 10-19 J
49
Autumn 2000 13. Hemoglobin absorbs light of
wavelength 407 nm. Calculate the energy (in J)
of one millimole of photons of this light. E
hn hc/l 6.626 x 10-34 x 3 x 108 /407 x 10-9
J s m s-1 m-1 4.88 x 10-19 J 1 millimole
10-3 mole 6.022 x 1020 photons
50
Autumn 2000 13. Hemoglobin absorbs light of
wavelength 407 nm. Calculate the energy (in J)
of one millimole of photons of this light. E
hn hc/l 6.626 x 10-34 x 3 x 108 /407 x 10-9
J s m s-1 m-1 4.88 x 10-19 J 1 millimole
10-3 mole 6.022 x 1020 photons energy of 1
millimole of photons ? 6.022 x 1020x 4.88 x 10-19
J
51
Autumn 2000 13. Hemoglobin absorbs light of
wavelength 407 nm. Calculate the energy (in J)
of one millimole of photons of this light. E
hn hc/l 6.626 x 10-34 x 3 x 108 /407 x 10-9
J s m s-1 m-1 4.88 x 10-19 J 1 millimole
10-3 mole 6.022 x 1020 photons energy of 1
millimole of photons ? 6.022 x 1020x 4.88 x 10-19
J 294 J
52
(No Transcript)
53
Line spectrum of sodium
400 550 700 nm
Line spectrum of hydrogen
400 550 700 nm
Balmer-Rydberg equation 1/l R1/m2 -
1/n2 m 1, n gt 1
54
- Quantization of e- energy levels by standing
- wave assumption
- Derived a mathematical description for e-s as
- 3-d standing waves in atoms
- H? E?
- ?, the wave function, is a function spatial
- position of e-
- H, is a mathematical function which allows the
- calculation of the e- energy (Hamiltonian
- operator) and
- E, is the total energy of the electron
55
What is ?2? " Amplitude probability function
" e.g. for a linear standing wave Amplitude
sin x , where x is distance along the wave
56
What is ?2? " Amplitude probability function
" e.g. for a linear standing wave Amplitude
sin x , where x is distance along the wave Why
amplitude probability function ? Heisenberg
Uncertainty Principle (1927)
57
What is ?2? " Amplitude probability function
" e.g. for a linear standing wave Amplitude
sin x , where x is distance along the wave Why
amplitude probability function ? Heisenberg
Uncertainty Principle (1927) "There is a
fundamental limit to how precisely we can
simultaneously determine both the position (x)
and the momentum (m.v) of a particle"
58
What is ?2? " Amplitude probability function
" e.g. for a linear standing wave Amplitude
sin x , where x is distance along the wave Why
amplitude probability function ? Heisenberg
Uncertainty Principle (1927) "There is a
fundamental limit to how precisely we can
simultaneously determine both the position (x)
and the momentum (m.v) of a particle" ?x.m?v gt
h/4? i.e. ?x.m?v gt 5.272(10-35) m2 kg s-1
59
Example For a ?v of 0.1m/s, calculate ?x for (a)
an electron (m9.11 x 10-31kg) and (b) a football
(m0.4kg).
60
Example For a ?v of 0.1m/s, calculate ?x for (a)
an electron (m9.11 x 10-31kg) and (b) a football
(m0.4kg). (a) ?x gt 5.272(10-35)/9.11(10-31)(
0.1) 5.79(10-4) m
61
Example For a ?v of 0.1m/s, calculate ?x for (a)
an electron (m9.11 x 10-31kg) and (b) a football
(m0.4kg). (a) ?x gt 5.272(10-35)/9.11(10-31)(
0.1) 5.79(10-4) m ?x of e- is big c.f. size
of an atom (2(10-10m))!
62
Example For a ?v of 0.1m/s, calculate ?x for (a)
an electron (m9.11 x 10-31kg) and (b) a football
(m0.4kg). (a) ?x gt 5.272(10-35)/9.11(10-31)(
0.1) 5.79(10-4) m ?x of e- is big c.f. size
of an atom (2(10-10m))! (b) ?x gt
5.272(10-35)/0.4(0.1) 1.31(10-33) m
63
Example For a ?v of 0.1m/s, calculate ?x for (a)
an electron (m9.11 x 10-31kg) and (b) a football
(m0.4kg). (a) ?x gt 5.272(10-35)/9.11(10-31)(
0.1) 5.79(10-4) m ?x of e- is big c.f. size
of an atom (2(10-10m))! (b) ?x gt
5.272(10-35)/0.4(0.1) 1.31(10-33) m ?x of
ball is v. small c.f. its size (0.3m)
64
Example For a ?v of 0.1m/s, calculate ?x for (a)
an electron (m9.11 x 10-31kg) and (b) a football
(m0.4kg). (a) ?x gt 5.272(10-35)/9.11(10-31)(
0.1) 5.79(10-4) m ?x of e- is big c.f. size
of an atom (2(10-10m))! (b) ?x gt
5.272(10-35)/0.4(0.1) 1.31(10-33) m ?x of
ball is v. small c.f. its size (0.3m) Conclusion
H.U.P. only important for v. small particles
such as e-
65
Major implication of H.U.P. It is not possible
to find out the exact position velocity of an
e- in an atom
66
Major implication of H.U.P. It is not possible
to find out the exact position velocity of an
e- in an atom Can only define the shapes of
electron "clouds" in terms of probability of
finding electron at a given spot
67
Major implication of H.U.P. It is not possible
to find out the exact position velocity of an
e- in an atom Can only define the shapes of
electron "clouds" in terms of probability of
finding electron at a given spot Probability or
e- density is given by the term ?2
68
Major implication of H.U.P. It is not possible
to find out the exact position velocity of an
e- in an atom Can only define the shapes of
electron "clouds" in terms of probability of
finding electron at a given spot Probability or
e- density is given by the term ?2 Probability
maps for a particular wavefunction are called
electron orbitals
69
Major implication of H.U.P. It is not possible
to find out the exact position velocity of an
e- in an atom Can only define the shapes of
electron "clouds" in terms of probability of
finding electron at a given spot Probability or
e- density is given by the term ?2 Probability
maps for a particular wavefunction are called
electron orbitals Orbitals usually drawn as
shape which encloses 90 of the total e- density
for that wavefunction
70
- Characterisation of Electron Orbitals
- Many different solutions to the Schroedinger
- Equation for an electron in an atom.
71
- Characterisation of Electron Orbitals
- Many different solutions to the Schroedinger
- Equation for an electron in an atom.
- Each solution represented by an orbital
72
- Characterisation of Electron Orbitals
- Many different solutions to the Schroedinger
- Equation for an electron in an atom.
- Each solution represented by an orbital
- A series of quantum numbers are used to
- describe the various properties of an orbital
73
- Characterisation of Electron Orbitals
- Many different solutions to the Schroedinger
- Equation for an electron in an atom.
- Each solution represented by an orbital
- A series of quantum numbers are used to
- describe the various properties of an orbital
- Principle quantum number (n) has integral values
- (1,2,3, etc.) describes orbital size and energy
74
- Characterisation of Electron Orbitals
- Many different solutions to the Schroedinger
- Equation for an electron in an atom.
- Each solution represented by an orbital
- A series of quantum numbers are used to
- describe the various properties of an orbital
- Principle quantum number (n) has integral values
- (1,2,3, etc.) describes orbital size and energy
- Angular momentum quantum number (l) has
- integral values from 0 to n-1 for each value of n
- describes the orbital shape
75
Magnetic quantum number (ml) has integral
values between l and -l, including zero
describes the orientation in space of the orbital
relative to the other orbitals in the atom
76
Magnetic quantum number (ml) has integral
values between l and -l, including zero
describes the orientation in space of the orbital
relative to the other orbitals in the atom
Spin quantum number (ms) is either 1/2 or -1/2
for a given e- describes the direction of spin
of the e- on its axis
77
Magnetic quantum number (ml) has integral
values between l and -l, including zero
describes the orientation in space of the orbital
relative to the other orbitals in the atom
Spin quantum number (ms) is either 1/2 or -1/2
for a given e- describes the direction of spin
of the e- on its axis Pauli Exclusion Principle
"no two electrons in an atom can have the same
set of quantum numbers", or, only two electrons
(of opposite spin) per orbital.
78
Some Quantum Nos. Orbitals in the H Atom
Sub-shell No. of n l
Designation ml Orbitals No. of e-
79
Some Quantum Nos. Orbitals in the H Atom
Sub-shell No. of n l
Designation ml Orbitals No. of e- 1
0 1s 0 1 2
80
Some Quantum Nos. Orbitals in the H Atom
Sub-shell No. of n l
Designation ml Orbitals No. of e- 1
0 1s 0 1 2 2
0 2s 0 1 2 1 2p -1, 0,
1 3 6
81
Some Quantum Nos. Orbitals in the H Atom
Sub-shell No. of n l
Designation ml Orbitals No. of e- 1
0 1s 0 1 2 2
0 2s 0 1 2 1 2p -1, 0,
1 3 6 3 0 3s 0 1 2 1
3p -1, 0, 1 3 6 2 3d -2, -1, 0,
1, 2 5 10 4...etc...
82
(No Transcript)
83
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms 1/2
84
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms
1/2 invalid l 0 to n-1 (b) n 2, l 1, ml
0, ms 1/2 valid
85
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms
1/2 invalid l 0 to n-1 (b) n 2, l 1, ml
0, ms 1/2 valid (c) n 2, l 0, ml
-1, ms 1/2 invalid ml -l to l
86
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms
1/2 invalid l 0 to n-1 (b) n 2, l 1, ml
0, ms 1/2 valid (c) n 2, l 0, ml
-1, ms 1/2 invalid ml -l to l (d) n 3,
l 1, ml 0, ms 0 invalid ms -1/2 or
1/2
87
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms
1/2 invalid l 0 to n-1 (b) n 2, l 1, ml
0, ms 1/2 valid (c) n 2, l 0, ml
-1, ms 1/2 invalid ml -l to l (d) n 3,
l 1, ml 0, ms 0 invalid ms -1/2 or
1/2 Write a valid set of quantum numbers for
each of the following sub-shells (a) 2s n
2, l 0, ml 0, ms 1/2
88
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms
1/2 invalid l 0 to n-1 (b) n 2, l 1, ml
0, ms 1/2 valid (c) n 2, l 0, ml
-1, ms 1/2 invalid ml -l to l (d) n 3,
l 1, ml 0, ms 0 invalid ms -1/2 or
1/2 Write a valid set of quantum numbers for
each of the following sub-shells (a) 2s n
2, l 0, ml 0, ms 1/2 (b) 2p n 2, l
1, ml -1, ms 1/2
89
Which of the following are valid sets of quantum
numbers? (a) n 1, l 1, ml 0, ms
1/2 invalid l 0 to n-1 (b) n 2, l 1, ml
0, ms 1/2 valid (c) n 2, l 0, ml
-1, ms 1/2 invalid ml -l to l (d) n 3,
l 1, ml 0, ms 0 invalid ms -1/2 or
1/2 Write a valid set of quantum numbers for
each of the following sub-shells (a) 2s n
2, l 0, ml 0, ms 1/2 (b) 2p n 2, l
1, ml -1, ms 1/2 (c) 3d n 3, l 2, ml
-2, ms 1/2
90
Principal quantum number specifies energy of e-
91
Principal quantum number specifies energy of
e- For hydrogen, Schroedinger showed that E
- hR/n2 where R is the Rydberg constant 3.29 X
1015 Hz
92
- Principal quantum number specifies energy of e-
- For hydrogen, Schroedinger showed that
- E - hR/n2
- where R is the Rydberg constant 3.29 X 1015 Hz
- Can calculate from theory the hydrogen spectrum
93
- Principal quantum number specifies energy of e-
- For hydrogen, Schroedinger showed that
- E - hR/n2
- where R is the Rydberg constant 3.29 X 1015 Hz
- Can calculate from theory the hydrogen spectrum
- For transition from n 3 to n 2 state
94
- Principal quantum number specifies energy of e-
- For hydrogen, Schroedinger showed that
- E - hR/n2
- where R is the Rydberg constant 3.29 X 1015 Hz
- Can calculate from theory the hydrogen spectrum
- For transition from n 3 to n 2 state
- DE -hR1/32 - 1/22 - h x 3.29 x 10151/9 -
1/4
95
- Principal quantum number specifies energy of e-
- For hydrogen, Schroedinger showed that
- E - hR/n2
- where R is the Rydberg constant 3.29 X 1015 Hz
- Can calculate from theory the hydrogen spectrum
- For transition from n 3 to n 2 state
- DE -hR1/32 - 1/22 - h x 3.29 x 10151/9 -
1/4 - h x 4.57 x 1014 Hz hn and n 4.57 x 1014 Hz
96
- Principal quantum number specifies energy of e-
- For hydrogen, Schroedinger showed that
- E - hR/n2
- where R is the Rydberg constant 3.29 X 1015 Hz
- Can calculate from theory the hydrogen spectrum
- for transition from n 3 to n 2 state
- DE -hR1/32 - 1/22 - h x 3.29 x 10151/9 -
1/4 - h x 4.57 x 1014 Hz hn and n 4.57 x 1014 Hz
- l c/n 3 x 108 ms-1/ 4.57 x 1014 s-1 656 nm
97
Line spectrum of hydrogen
400 550 700 nm
98
- For hydrogen, E - hR/n2
- If H atom acquires enough energy e- can be
promoted from - one level to the next
99
- For hydrogen, E - hR/n2
- If H atom acquires enough energy e- can be
promoted from - one level to the next
- To promote an e- from n 1 to n 2 level
- DE -hR1/22 - 1/12 - h x 3.29 x 10151/4 -
1 1.63 x 10-18 J
100
- For hydrogen, E - hR/n2
- If H atom acquires enough energy e- can be
promoted from - one level to the next
- To promote an e- from n 1 to n 2 level
- DE -hR1/22 - 1/12 - h x 3.29 x 10151/4 -
1 1.63 x 10-18 J - To promote an e- from n 2 to n 3 level
- DE -hR1/32 - 1/22 - h x 3.29 x 10151/9 -
1/4 3.03 x 10-19 J
101
- For hydrogen, E - hR/n2
- If H atom acquires enough energy e- can be
promoted from - one level to the next
- To promote an e- from n 1 to n 2 level
- DE -hR1/22 - 1/12 - h x 3.29 x 10151/4 -
1 1.63 x 10-18 J - To promote an e- from n 2 to n 3 level
- DE -hR1/32 - 1/22 - h x 3.29 x 10151/9 -
1/4 3.03 x 10-19 J - To remove e- (ionise) from the atom
- DE -hR1/?2 - 1/12 hR 2.18 x 10-18 J
102
- For hydrogen, E - hR/n2
- If H atom acquires enough energy e- can be
promoted from - one level to the next
- To promote an e- from n 1 to n 2 level
- DE -hR1/22 - 1/12 - h x 3.29 x 10151/4 -
1 1.63 x 10-18 J - To promote an e- from n 2 to n 3 level
- DE -hR1/32 - 1/22 - h x 3.29 x 10151/9 -
1/4 3.03 x 10-19 J - To remove e- (ionise) from the atom
- DE -hR1/?2 - 1/12 hR 2.18 x 10-18 J
- To ionise one mole of hydrogen atoms
103
Principle quantum number n 1, 2, 3,..
describes orbital size and
energy Angular momentum quantum number l 0 to
n-1 describes orbital shape
Magnetic quantum number ml l, l-1-l
describes orientation in space
of the orbital relative to the other
orbitals in the atom
Spin quantum number ms 1/2 or -1/2
describes the direction of spin of
the e- on its axis Pauli Exclusion Principle
"no two electrons in an atom can have the same
set of quantum numbers", or, only two electrons
(of opposite spin) per orbital.
104
Write a valid set of quantum numbers for each of
the following sub-shells (a) 2 s n 2, l
0, ml 0, ms - 1/2 n 2, l 0, ml 0, ms
1/2 2 combinations
105
Write a valid set of quantum numbers for each of
the following sub-shells (a) 2 s n 2, l
0, ml 0, ms - 1/2 n 2, l 0, ml 0, ms
1/2 2 combinations (b) 2 p n 2, l 1,
ml -1, ms - 1/2 n 2, l 1, ml -1, 0
or 1, ms 1/2 6 combinations
106
Write a valid set of quantum numbers for each of
the following sub-shells (a) 2 s n 2, l
0, ml 0, ms - 1/2 n 2, l 0, ml 0, ms
1/2 2 combinations (b) 2 p n 2, l 1,
ml -1, ms - 1/2 n 2, l 1, ml -1, 0
or 1, ms 1/2 6 combinations (c) 3 d n
3, l 2, ml -2, ms - 1/2 n 3, l 2, ml
-2, -1, 0, 1, or 2, ms 1/2 10 combinations
107
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5
108
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5 2 l 1
orbitals per subshell
109
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5 2 l 1
orbitals per subshell How many orbitals in a
shell? n 1, 1s 1 n 2, 2s, 2px, 2py,
2pz 4 n 3, 3s, 3px, 3py, 3pz, 3dxy,,
3dxz,, 3dyz ,, 3dx2-y2, 3dz2 9
110
How many orbitals in a subshell? l 0,
1s 1 l 1, px, py, pz 3 l 2,
dxy,, dxz,, dyz ,, dx2-y2, dz2 5 2 l 1
orbitals per subshell How many orbitals in a
shell? n 1, 1s 1 n 2, 2s, 2px, 2py,
2pz 4 n 3, 3s, 3px, 3py, 3pz, 3dxy,,
3dxz,, 3dyz ,, 3dx2-y2, 3dz2 9 n2 orbitals per
principal quantum level
111
- Hydrogen atom-
- all orbitals within a shell have the same energy
- electrostatic interaction between e- and proton
112
- Hydrogen atom-
- all orbitals within a shell have the same energy
- electrostatic interaction between e- and proton
- Multi-electron atoms-
- the energy level of an orbital depends not only
on the - shell but also on the subshell
- electrostatic interactions between e- and proton
and other e-
113
- Quantum Mechanical Model for Multi-electron Atoms
- electron repulsions
- He He e- E 2372 kJ mol-1
- He has two electron which repel each other
114
- Quantum Mechanical Model for Multi-electron Atoms
- electron repulsions
- He He e- E 2372 kJ mol-1
- He has two electron which repel each other
- He He2 e- E 5248 kJ mol-1
- He has one electron, no electrostatic repulsion
115
- Quantum Mechanical Model for Multi-electron Atoms
- electron repulsions
- He He e- E 2372 kJ mol-1
- He has two electron which repel each other
- He He2 e- E 5248 kJ mol-1
- He has one electron, no electrostatic repulsion
- Less energy required to remove e- from He than
from He - Shielding of outer orbital electrons from ve
nuclear - charge by inner orbital electrons
- gt outer orbital electrons have higher energies
116
- Quantum Mechanical Model for Multi-electron Atoms
- Penetration effect of outer orbitals within
inner orbitals - ns gt np gt nd
- For a given n, energy of s lt energy of p lt energy
of d
117
- Quantum Mechanical Model for Multi-electron Atoms
- Penetration effect of outer orbitals within
inner orbitals - ns gt np gt nd
- For a given n, energy of s lt energy of p lt energy
of d - Effective nuclear charge (Zeff) experienced by
an electron - is used to quantify these additional effects.
118
- Quantum Mechanical Model for Multi-electron Atoms
- Penetration effect of outer orbitals within
inner orbitals - ns gt np gt nd
- For a given n, energy of s lt energy of p lt energy
of d - Effective nuclear charge (Zeff) experienced by
an electron - is used to quantify these additional effects.
- Example Sodium, Na, Z 11
- Na 1s e- Zeff 10.3 shielding
effect is small - Na 3s e- Zeff 1.84 large
shielding effect by inner e-s - penetration effect counteracts
- this to a small extent
119
Orbital Energies
3dxy
3dxz
3dyz
3dx2-y2
3dz2
3px
3py
3pz
3s
Energy
2px
2py
2pz
2s
1s
120
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle
121
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle)
122
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
123
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
H (Z 1) 1s1
2s 2p
Energy
1s
124
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
N (Z 7) 1s2, 2s2, 2p3,
2p
2s
Energy
1s
125
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
B (Z 5) 1s2, 2s2, 2p1
2p
2s
Energy
1s
126
Electronic Configuration Filling-in of Atomic
Orbitals Rules 1. Pauli Principle 2. Fill
in e-'s from lowest energy orbital upwards
(Aufbau Principle) 3. Try to attain maximum
number of unpaired e- spins in a given
sub-shell (Hund's Rule)
F (Z 9) 1s2, 2s2, 2p5
2p
2s
Energy
1s
127
Hydrogen 2s 3s 4s 1s
2p 3p 4p 3d 4d 4f Multi-electron
atoms 1s 2s 3s 4s 5
s 2p 3p 4p 3d 4d
128
1s 2s 2px 2py 2pz
H 1s1 He 1s2 Li 1s2, 2s1 Be 1s2,
2s2 B 1s2, 2s2, 2px1 C 1s2, 2s2, 2px1, 2py1 N
1s2, 2s2, 2px1, 2py1, 2pz1 O 1s2, 2s2,
2px2, 2py1, 2pz1 F 1s2, 2s2, 2px2, 2py2,
2pz1 Ne 1s2, 2s2, 2px2, 2py2, 2pz2
129
H 1s1 He 1s2 Li He, 2s1 Be He, 2s2
130
H 1s1 He 1s2 Li He, 2s1 Be He,
2s2 B He, 2s2, 2p1 Ne He, 2s2, 2p6 Na
He, 2s2, 2p6, 3s1 ? Ne, 3s1
131
H 1s1 He 1s2 Li He, 2s1 Be He,
2s2 B He, 2s2, 2p1 Ne He, 2s2, 2p6 Na
He, 2s2, 2p6, 3s1 ? Ne, 3s1 Mg He, 2s2,
2p6, 3s2 ? Ne, 3s2 Al Ne, 3s2, 3p1 Si
Ne, 3s2, 3p2
132
H 1s1 He 1s2 Li He, 2s1 Be He,
2s2 B He, 2s2, 2p1 Ne He, 2s2, 2p6 Na
He, 2s2, 2p6, 3s1 ? Ne, 3s1 Mg He, 2s2,
2p6, 3s2 ? Ne, 3s2 Al Ne, 3s2, 3p1 Si
Ne, 3s2, 3p2 P Ne, 3s2, 3p3 S Ne, 3s2,
3p4 Cl Ne, 3s2, 3p5 Ar Ne, 3s2, 3p6
133
- H 1s1 He 1s2
- Li He, 2s1 Be He, 2s2
- B He, 2s2, 2p1 Ne He, 2s2, 2p6
- Na He, 2s2, 2p6, 3s1 ? Ne, 3s1
- Mg He, 2s2, 2p6, 3s2 ? Ne, 3s2
- Al Ne, 3s2, 3p1 Si Ne, 3s2, 3p2
- P Ne, 3s2, 3p3 S Ne, 3s2, 3p4
- Cl Ne, 3s2, 3p5 Ar Ne, 3s2, 3p6
- outermost shell - valence shell
- most loosely held electron and are the most
important - in determining an elements properties
134
K Ar, 4s1 Ca Ar, 4s2 Sc Ar, 4s2, 3d1
Ca Ar, 4s2, 3d2
135
K Ar, 4s1 Ca Ar, 4s2 Sc Ar, 4s2, 3d1
Ca Ar, 4s2, 3d2 Zn Ar, 4s2, 3d10 Ga
Ar, 4s2, 3d10, 3p1 Kr Ar, 4s2, 3d10, 3p6
136
K Ar, 4s1 Ca Ar, 4s2 Sc Ar, 4s2, 3d1
Ca Ar, 4s2, 3d2 Zn Ar, 4s2, 3d10 Ga
Ar, 4s2, 3d10, 3p1 Kr Ar, 4s2, 3d10,
3p6 Anomalous electron configurations d5 and
d10 are lower in energy than expected Cr
Ar, 4s1, 3d5 not Ar, 4s2, 3d4
Cu Ar, 4s1, 3d10 not Ar, 4s2, 3d9
137
Electron Configuration of Ions Electrons lost
from the highest energy occupied orbital of the
donor and placed into the lowest unoccupied
orbital of the acceptor (placed according to the
Aufbau principle)
138
Electron Configuration of Ions Electrons lost
from the highest energy occupied orbital of the
donor and placed into the lowest unoccupied
orbital of the acceptor (placed according to the
Aufbau principle) Examples Na Ne,
3s1 Na Ne e- Cl Ne, 3s2, 3p5 e-
Cl- Ne, 3s2, 3p6 Mg Ne, 3s2 Mg2
Ne O He, 2s2, 2p4 O2- He, 2s2, 2p6
139
- Modern Theories of the Atom - Summary
- Wave-particle duality of light and matter
- Bohr theory
- Quantum (wave) mechanical model
- Orbital shapes and energies
- Quantum numbers
- Electronic configuration in atoms