Formic acid: HCOOH - PowerPoint PPT Presentation
Title:
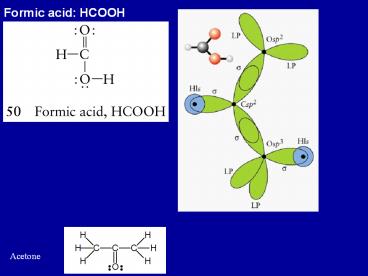
Formic acid: HCOOH
Description:
Formic acid: HCOOH. Acetone. Benzene C6H6. Resonance structures; each point corresponds to a CH ... Each C is sp2 hybridized, one of the sp2 forming a s-bond ... – PowerPoint PPT presentation
Number of Views:2572
Avg rating:3.0/5.0
Title: Formic acid: HCOOH
1
- Formic acid HCOOH
Acetone
2
- Benzene C6H6
Kekulé structures
Resonance structures each point corresponds to a
CH Each C is sp2 hybridized, one of the sp2
forming a s-bond with H 1s orbital and the other
two forming s-bonds with adjacent C sp2
orbitals.
3
- The un-hybridized p orbital on each C is
available for p-bonding with p orbitals on either
of the adjacent C atoms
4
- Actual structure of benzene is a resonance hybrid
of the two alternating bond patterns the 6 C
atoms are identical, and the electrons in the
p-bonds spread around the entire ring - This lowers the energy of the molecule -
resonance adds stability to a molecule
5
Characteristics of p bonds bonds Energy of CC
is lt 2 x energy of C-C bond Energy of C?C is lt 3
x energy of C-C bond C, N, O form double bonds
with one another and with elements from later
periods Double bonds are rarely found between
elements in period 3 are below - atoms are too
large for effective side-by-side
overlap. Molecules with alternate double-single
bonds - conjugated molecules
6
Isomers Molecules with the same molecular
formula but different structures
cis-1,2-dichloroethylene
trans-1,2-dichloroethylene
Rotation can occur about a single sigma
bond Rotation is restricted about a double bond
isomers are a consequence
7
Change of shape triggers a signal along the optic
nerve
8
Molecular Orbital Theory
- VB theory localized bond
- VB theory provides the basis of calculating
electron distributions in molecules but cannot
explain the properties of some molecules. - O2
- VB theory
- O Is2 2s2 2p4
- sp2 hybridized O, one sp2 from each forms s-bond
and the other two are occupied with the lone
pairs. - The un-hybridized p on each forms the p-bond
- Indicates that in O2 molecule, all electrons are
paired. - However O2 was observed to be paramagnetic
9
- VB theory assumes that the electrons are
localized between the two bonding atoms - Molecular orbital theory electrons are spread
throughout the entire molecule electrons are
delocalized over the whole molecule. - Pure atomic orbitals combine to produce molecular
orbitals that are spread out, delocalized, over
an entire molecule - Molecular orbitals are built by adding together
-superimposing - atomic orbitals belonging to the
valence shell of the atoms in the molecules.
10
- H2 wavefunction representing the molecular
orbitals (MOs) for H2 can be represented by
combining the two atomic orbitals (AOs) for the
separated H atoms. - Wavefunction of the H2 MO
- y yA1s yB1s
- yA1s or yB1s 1s orbital centered on one of the H
atom(A or B) - The molecular orbital, y, is a linear combination
of atomic orbitals - Any molecular orbital formed from a superposition
of atomic orbitals is called a LCAO-MO. - y is a bonding orbital energy of y is lower
than that of either AO - In H2, the contribution from each AO to the MO is
equal
11
- The two AOS are waves centered on different
nucleii. - Bonding orbital AO wavefunctions interfere
constructively - MO wavefunction in blue.
12
- N AOs overlapping will form N MOs
- Two H AOs overlapping form two Mos one of which
is the bonding orbital, y. - The wavefunctions of the two H AOs can also
interfere destructively - anti-bonding MO of
higher energy than each of the AOs - y- yA1s - yB1s
- Node between two nuclei
Probability of finding electrons between nuclei
reduced nuclei repel each other
http//www.shef.ac.uk/chemistry/orbitron/index.htm
l
13
(No Transcript)
14
Molecular Orbital Energy Level Diagram
Energy of bonding MO lt AO Energy of
anti-bonding MO gt AO
15
- Diatomic Molecules
- Build all possible MOs from available valence AOs
- Then accommodate valence electrons in molecular
orbitals using the aufbau principles - 1) Electrons occupy the lowest energy MOs first,
then orbitals of increasing energy - 2) Pauli exclusion principle each orbital can
occupy up to two electrons if two electrons in
an orbital must be paired - 3) Hunds rule if more than one orbital of the
same energy is available electrons enter them
singly with parallel spinds.
16
Lowest unoccupied MO (LUMO)
Highest occupied MO (HOMO)
- H2 molecular orbital energy-level diagram or
correlation diagram - Bonding MO - s1s anti-bonding MO s 1s
H2 Ground state electron configuration (s1s)2
17
- Bond Order
- 0.5(number of electrons in bonding MOs
- - number of electrons in anti-bonding MOs)
H2 bond order 0.5 (s1s)1
H2 bond order 1 (s1s)2
He2 bond order 0 (s1s)2 (s1s)2
18
- Period 2 elements
- In period 2 elements each atom has one 2s and
three 2p valence AOs expect to form eight MOs - The two 2s orbitals (one from each atom) overlap
to form a s2s bonding MO and a s2s antibonding
MO - The six 2p orbitals (three from each atom)
overlap to form six MOs - The two 2p-orbitals directed toward each other
form a bonding s-orbital (s2p) and an
anti-bonding s-orbital (s2p) - Two 2p orbitals that are perpendicular to the
internuclear axis overlap side by side to form
two bonding p and two anti-bonding p orbitals.
19
Anti-bonding
Bonding
- s and s orbitals formed from p AOs
p and p orbitals formed from p AOs
20
MO diagram for homonuclear diatomic molecules O2
and F2
- MO diagram for homonuclear diatomic molecules Li2
through N2